|
Class 9 Hydrogen Exercise 6(A) Chapter-6
Question 1
Justify the Position of Hydrogen in the periodic
table.
Answer 1
Hydrogen is the first element in the periodic table. Its
atomic number is 1, and it has only one electron in its
valence shell. So, it belongs to the first group and the
first period of the periodic table.
Question 2
Why does hydrogen show dual nature?
Answer 2
Hydrogen shows dual nature because it resembles the alkali
metals of Group IA and the halogens of Group VIIA.
Question 3
Compare hydrogen with alkali metals on basis of:
(i) Ion formation
(ii) Reducing power
(iii) Reaction with oxygen
(iv) Oxide formation
Answer 3
(i) Each of them can form a cation by loss of an electron.
H → H+ + e–
Li →Li+ + e–
(ii) Both alkali metals and hydrogen act as reducing agents.
CuO + H2 → Cu + H2O
CuO + Na → Cu + Na2O
(iii) Hydrogen burns in oxygen to form its oxide. It burns
with a pop sound.
2H2 + O2 → 2H2O
Alkali metals also burn vigorously when heated in oxygen to
form their respective oxides.
Lithium forms monoxide.
4Li + O2 → 2Li2O
(iv) Hydrogen burns in oxygen to form its oxide. It burns
with a pop sound.
2H2 + O2 → 2H2O
Alkali metals also burn vigorously when heated in oxygen to
form their respective oxides.
Lithium forms monoxide.
4Li + O2 → 2Li2O
Question 4
In what respect does hydrogen differ from:
(i) alkali metals
(ii) halogens?
Answer 4
(i) Oxides of alkali metals are basic in nature, whereas the
oxide of hydrogen H2O is a neutral oxide.
(ii)Hydrogen atom has only one shell, but halogens have two
or more shells.
Question 5
Give the general group study of hydrogen with reference to
(i) valence electrons
(ii) burning
(iii) reducing power
Answer 5
(i) Hydrogen has one valence electron in its outermost
orbit.
(ii) Hydrogen burns in oxygen to form its oxide. It burns
with a pop sound.
2H2 + O2 → 2H2O
(iii) Hydrogen acts as a reducing agent.
CuO + H2 → Cu + H2O
Question 6
Why hydrogen was called ‘inflammable air’?
Answer 6
Hydrogen was called inflammable air because of its
combustible nature.
Question 7
State some sources of hydrogen.
Answer 7
In the free state, hydrogen is found in traces in the
earth’s crust and atmosphere.
And In the combined state, plant and animal tissues are made
of compounds of hydrogen with carbon, oxygen and nitrogen.
Question 8
Compare hydrogen and halogens on the basis of:
(i) physical state
(ii) ion formation
(iii) valency
(iv) reaction with oxygen
Answer 8
(i) Like halogens (fluorine and chlorine), hydrogen too is a
gas.
(ii) Both show a tendency to form anions because they are
one electron short of the nearest inert gas configuration.
H + e– → H–
Cl + e– →Cl–
(iii) Both have valency 1.
(iv) Hydrogen reacts with oxygen to form neutral oxide, H2O.
Halogens react with oxygen to form acidic oxides like Cl2O
and Cl2O7.
Question 9
Which metal is preferred for preparation of hydrogen.
(i) from water?
(ii) from acid?
Answer 9
(i) Reactive metals such as potassium, sodium and calcium.
(ii) Magnesium, aluminium, zinc and iron.
Question 10
(i) Write the reaction of steam with red hot iron.
(ii) Why this reaction is considered as reversible reaction?
(iii) How the reaction can proceed continuously?
Answer 10
(i) 3Fe + 4H2O
⇋ Fe3O4 + 4H2
(ii) The reaction is reversible because if hydrogen formed
is not removed, then the iron oxide formed is reduced back
to iron.
(iii) Because the reaction is a reversible reaction,
equilibrium is attained at 700°C. At this stage, the amount
of reactants and products does not change.
Question 11
why zinc and aluminium are considered to have unique nature.
Give balanced equations to support your explanation.
Answer 11
They react with acids and can even react with hot
concentrated alkalis to form hydrogen and a soluble salt.
Zn + 2NaOH → Na2ZnO2 + H2
2Al + 6NaOH→ 2Na2Al O3 + 3H2
Oxides and hydroxides of zinc and aluminium are amphoteric.
They react with both bases and acids to give salt and water.
ZnO + 2HCl → ZnCl2 + H2O
ZnO + 2NaOH →Na2ZnO2 + H2O
Question 12
Write balanced equations for the following:
(i) Iron reacts with dil. HCl
(ii) Zinc reacts with caustic soda solution
(iii) Lead reacts with potassium hydroxide
(iv) Aluminium reacts with fused sodium hydroxide
Answer 12
(i) Fe +2 HC l → FeCl2 + H2
(ii) Zn + 2NaOH → Na2ZnO2 + H2
(iii) Pb + 2KOH → K2PbO2 + H2
(iv) 2Al + 6NaOH→ 2Na2Al O3 + 3H2
Question 13
Write the balanced equations and give your observations when
the following metals react:
(i) Sodium with cold water
(ii) Calcium with cold water
(iii) Magnesium with boiling water
(iv) Magnesium with steam
Answer 13
(i) The reaction is highly exothermic and vigorous with the
evolution of hydrogen.
2Na + 2H2O →2NaOH + H2
(ii) Calcium sinks in water and the reaction is less
vigorous.
Ca + 2H2O → Ca(OH)2 + H2
(iii) Magnesium reacts slowly with boiling water and forms a
base, magnesium hydroxide, liberating hydrogen gas.
Mg + 2H2O →Mg(OH)2 + H2O
(iv) Magnesium burns in steam with an intense white light
liberating hydrogen gas and white ash, i.e. magnesium oxide.
Mg + H2O → MgO + H2
Question 14
(i) Under what conditions iron reacts with water.
(ii) Give the balanced equation of the reaction.
(iii) What is noticed if the products are not allowed to
escape?
Answer 14
(i) Iron is less reactive than zinc, but red hot iron reacts
with steam, forming triferric tetra-oxide and hydrogen gas.
(ii) 3Fe + 4H2O
⇋ Fe3O4 + 4H2
(iii) If the product formed, i.e. hydrogen is not removed,
then the iron oxide formed is reduced back to iron.
Question 15
From the knowledge of activity series, name a metal which
shows the following properties
(i) It reacts readily with cold water.
(ii) It displaces hydrogen from hot water.
(iii) It displaces hydrogen from dilute HCl.
(iv) It forms a base which is insoluble in water.
Answer 15
(i) Sodium
(ii) Magnesium
(iii) Zinc
(iv) Calcium
Question 16
Complete the following word equations:
(i) Sodium hydroxide + zinc → hydrogen + _________
(ii) Calcium + water → calcium hydroxide + _________
Answer 16
(i) Sodium hydroxide + zinc → hydrogen + sodium zincate
(ii) Calcium + water → calcium hydroxide + hydrogen
Chapter-6 Hydrogen Study
of First Element Selina Solutions Exercise – 6(B)
Question 1
Hydrogen can be prepared with the metal zinc by using:
(i) acid
(ii) alkali
(iii) water
Give an equation in each case.
Answer 1
(i) Zn + HCl → ZnCl2 + H2
(ii) Zn + 2NaOH → Na2ZnO2 + H2
(iii) Zn + H2O → ZnO + H2
Question 2
For laboratory preparation of hydrogen, give the following:
(a) materials used
(b) method of collection
(c) chemical equation
(d) fully-labelled diagram
Answer 2
(a) Granulated zinc, dilute HCl or dil. H2SO4
(b) It is collected by the downward displacement of water.
(c) Zn + HCl → ZnCl2 + H2
(d)
Question 3
(a) Name the impurities present in hydrogen prepared in the
laboratory.
(b) How can these impurities be removed?
Answer 3
(a) Hydrogen sulphide, sulphur dioxide, oxides of nitrogen,
phosphine, arsine, carbon dioxide and watervapour are
impurities present in the laboratory.
(b) The impurities can be removed from hydrogen by passing
it through
Silver nitrate solution to remove arsine and phosphine.
Lead nitrate solution to remove hydrogen sulphide.
Pb(NO3)2
+ H2S → PbS + 2HNO3
Caustic potash solution to remove sulphur dioxide, carbon
dioxide and oxides of nitrogen.
SO2 + 2KOH → K2SO3
+ H2O
CO2 + 2KOH→ K2CO3+
H2O
2NO2 + 2KOH
→KNO2 + KNO3 + H2O
A drying agent used to dry the gas. Common drying agents
such as fused calcium chloride, caustic potash stick and
phosphorus pentoxide remove water vapour.
So, the gas is purified and dried and then collected over
mercury because mercury does not react with it.
Question 4
Which test should be made before collecting hydrogen in a
gas jar?
Answer 4
Test: Collect some amount of gas in a test tube and take it
to a flame.
If the gas burns quietly, then there is no more air in the
flask.
Question 5
Why nitric acid is not used in the preparation of hydrogen?
Answer 5
Nitric acid is a powerful oxidising agent, and the oxygen
formed due to its decomposition oxidiseshydrogen to give
water thus defeating the purpose of the reaction.
3Zn + 8HNO3 →
3Zn(NO3)2 + 4H2O + 2NO
Question 6
Why hot concentrated sulphuric acid is not used in the
preparation of hydrogen?
Answer 6
Conc. sulphuric acid is not used in the preparation of
hydrogen as it will produce sulphur dioxide.
Zn + 2H2SO4 →ZnSO4 + SO2
+ 2H2O
Question 7
Hydrogen is manufactured by ‘Bosch Process’.
(a) Give the equations with conditions.
(b) How can you obtain hydrogen from a mixture of hydrogen
and carbon monoxide?
Answer 7
(a) C + H2O→ (CO + H2) – ∆
(b) (CO + H2)+ H2O→CO2 + 2H2
+ ∆
The mixture is passed through ammoniacal cuprous chloride
solution in order to dissolve any uncombined carbon
monoxide.
CuCl + CO + 2H2O →CuCl.CO.2H2O
Question 8
Give equations to express the reaction between:
(a) Steam and red hot iron
(b) Calcium and water
Answer 8
(a) 3Fe + 4H2O⇋ Fe3O4 + 4H2
(b) Ca + 2H2O
⇋
Ca(OH)2 + H2
Question 9
A small piece of calcium metal is put into a small trough
containing water. There is effervescence and white turbidity
is formed.
(a) Name the gas formed in the
reaction. How would you test the gas?
(b) Write an equation for the
reaction.
(c) What do you observe when a few
drops of red litmus solution are added to the turbid
solution.
Answer 9
Hydrogen gas. When red litmus is introduced in the solution,
it turns blue.
(a) Ca + 2H2O → Ca(OH)2
+ H2
(b) The solution turns blue.
(c) If dilute hydrochloric acid is
added to the turbid solution, then they react and neutralise
each other, forming the soluble salt calcium chloride (CaCl2)
and water.
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
Question 10
Thin strips of magnesium,copper and iron are taken.
(a) Write down what happens when
these metals are treated as follows:
(i) Heated in presence of air
(ii) Heated with dil.HCl
(iii)Added to an aqueous solution
of zinc sulphat
(b) Arrange these metals in
descending order of reactivity.
Answer 10
(a)
(i) On heating thin strips of
magnesium, copper and iron, they form oxides.
(ii) Magnesium and iron react with
HCl liberating hydrogen and forming their respective salts.
Hydrogen cannot be prepared from metals which are below it
in the activity series of metals (such as copper) because
only metals which are more reactive than hydrogen can
displace it from acids.
(iii) Only magnesium will displace
zinc from zinc sulphate solution because magnesium is more
reactive than zinc in the activity series of metals. No
reaction takes place in case of copper and iron because they
are less reactive than zinc.
(b)Mg > Fe > Cu
Question 11
Choose the correct option:
(a )Hydrogen is evolved by the
action of cold dil. HNO3 on
A. Fe B. Cu C. Mg D. Zn
(b) Which metal absorbs hydrogen?
A. Al B. Fe C. Pd D. K
(c)The composition of nucleus of
deuterium is
A. 1 e– and 1P B. 1 P and 1 A
C. 1 n and 1 e– D. 2P and 1 e–
(d) Elements which show unique
nature in the preparation of hydrogen are:
A. Na, K, Li B. Mg, Ca, Ba
C. Al, Zn, Pb D. Fe, Cu, Ag
Answer 11
(a) C. Mg
(b) C. Pd
(c) C. 1 n and 1 e–
(d) C. Al, Zn, Pb
Question 12
Give reasons for the following:
(a) Zinc granules are used in lab
preparation of hydrogen.
(b) Purified and dried hydrogen is
collected over mercury.
(c)The end of the thistle funnel
should be dipped under acid
(d) Dilute sulphuric acid cannot be
replaced by concentrated acid in the preparation of
hydrogen.
Answer 12
(a) Zinc granules are preferred
over pure zinc in the lab preparation of hydrogen because
the impurity present in granulated zinc is copper, whose
catalysing effect speeds up the rate of the reaction.
(b) Purified and dried hydrogen is
collected over mercury because mercury has no reaction with
it.
(c) The end of the thistle funnel
should be dipped under acid so as to prevent the gas from
escaping from the thistle funnel.
(d) Dilute sulphuric acid cannot be
replaced by concentrated acid in the preparation of hydrogen
because it is a strong oxidising agent and it will produce
sulphur dioxide.
Study of First Element-Hydrogen Exercise – 6(C)
Question 1
(a) Where does hydrogen occur in
free state?
(b) How did the name ‘hydrogen’
originate?
Answer 1
(a) In the free state, hydrogen is
found in traces in the earth’s crust and atmosphere.
Volcanic gases contain 0.025%, the earth’s crust 0.98%, the
earth’s atmosphere 0.01% and the atmosphere of the Sun and
stars 1.1%.
(b) The name ‘hydrogen’ originated
on account of its ability to form water.
Question 2
Hydrogen can be prepared with the help of cold water. Give a
reaction of hydrogen with:
(a) A monovalent metal
(b) A divalent metal
Answer 2
(a) 2K + 2H2O →2KOH + H2
(b) Ca + 2H2O → Ca(OH)2
+ H2
Question 3
Which metal is preferred for collecting hydrogen from:
(a) Cold water
(b) Hot water
(c) Steam
Answer 3
Metal preferred for collecting hydrogen from
(a) Cold water: Sodium
(b) Hot water: Magnesium
(c) Steam: Aluminium
Question 4
Hydrogen may be prepared in the laboratory by the action of
a metal on an acid.
(a) Which of the metals copper,
zinc, magnesium or sodium would be the most suitable?
(b) Which of the acids dilute
sulphuric, concentrated sulphuric, dilute nitric acid and
concentrated nitric acid would you choose? Explain why you
would not use the acids you reject.
(c) How would you modify your
apparatus to collect dry hydrogen? Which drying agent would
you employ for this purpose?
Answer 4
(a) Zinc is the most preferred
metal in the laboratory preparation of hydrogen.
(b) Dilute sulphuric acid.
Conc. nitric acid, even in its
dilute form, is not used in the preparation of hydrogen from
metals because it is a powerful oxidising agent and oxygen
formed due to its decomposition oxidiseshydrogen to give
water, thus defeating the purpose of the reaction.
Conc. sulphuric acid is not used in
the preparation of hydrogen as it will produce sulphur
dioxide.
(c) The gas is collected by the
downward displacement of water.
Common drying agents such as fused
calcium chloride, caustic potash stick and
phosphoruspentoxide remove water vapour.
Question 5
Why are the following metals not used in the lab.
preparation of hydrogen?
(a) calcium
(b) iron
(c) aluminium
(d) sodium
Answer 5
(a) Calcium is expensive.
(b) Iron has to be heated, and
hydrogen thus produced contains impurities such as hydrogen
sulphide and sulphur dioxide.
(c) Aluminium forms a protective
coating of Al2O3 due to its great
affinity for oxygen. So, it does not give hydrogen with acid
after the reaction has occurred for some time.
(d) Sodium reacts violently with
acid.
Question 6
Based on the reactions of water on
metals, arrange the following metals in increasing order of
reactivity: iron, sodium, magnesium, zinc, calcium
Answer 6
Increasing order of reactivity of
metals:
Iron < Zinc < Magnesium < Calcium <
Sodium
Question 7
Hydrogen is evolved when dilute HCl
reacts with magnesium, but nothing happens in the case of
mercury and silver. Explain.
Answer 7
Hydrogen is evolved when dilute HCl
reacts with magnesium which is placed above hydrogen in the
activity series. However, this does not occur for metals
below hydrogen such as mercury and silver. This is because
only metals which are more reactive than hydrogen can
displace it from HCl.
Question 8
Steam can react with metal and
non-metal to liberate hydrogen. Give necessary conditions
and equations for the same.
Answer 8
With metals:
3Fe + 4H2O
⇋
Fe3O4 + 4H2
With non-metals:
Steam is passed over hot coke
(1000°C) in furnaces of a special design called inverters
giving water gas.
C + H2O (CO + H2)
– ∆
Water is mixed with excess steam
and passed over heated ferric oxide which acts as a catalyst
and chromic oxide which acts as a promoter.
(CO + H2) + H2O
CO2 + 2H2 + ∆
The above mixture CO2 +
H2 is formed through cold water under pressure
(30 atm) or through caustic potash solution, which dissolves
the more soluble carbon dioxide leaving hydrogen.
2KOH + CO2 →K2CO3 + H2O
The mixture is passed through
ammoniacal cuprous chloride solution in order to dissolve
any uncombined carbon monoxide.
CuCl + CO + 2H2O →CuCl.CO.2H2O
Question 9
Hydrogen is obtained by
displacement from:
(a) dilute sulphuric acid
(b) dilute hydrochloric acid
Write equations using zinc and
iron.
Why does copper not show similar
behavior?
Answer 9
(a) Zn + H2SO4
→ ZnSO4 + H2
(b) Fe + H2SO4
→ FeSO4 + H2
Hydrogen cannot be prepared from
metals which are below it in the activity series of metals
such as copper because only metals which are more reactive
than hydrogen can displace it from acids.
Question 10
Give reason for the following:
(a) Though lead is above hydrogen
in the activity series, it does not react with dilute
hydrochloric acid or dilute sulphuric acid.
(b) Potassium and sodium are not
used for reaction with dilute hydrochloric acid or dilute
sulphuric acid in laboratory preparation of hydrogen.
Answer 10
(a) It forms an insoluble coating
of lead sulphate or lead chloride. So, further reaction is
prevented.
(b) Potassium and sodium react
violently with acid. Hence, potassium and sodium are not
used for reaction with dilute hydrochloric acid or dilute
sulphuric acid in the laboratory preparation of hydrogen.
Question 11
Name two alkalies that can displace
hydrogen. Give balanced equations for the same. Why are the
metals you have used considered to have unique nature?
Answer 11 two alkalies that can
displace hydrogen
NaOH and KOH
Zn + 2NaOH → Na2ZnO2
+ H2
Zn + 2KOH → K2ZnO2
+ H2
Metals such as zinc, lead and
aluminium have a unique nature. They react with acids and
can even react with hot alkalis to form hydrogen and a
soluble salt.
Question 12
Complete and balance the following
reactions.
(a) Na + H2O
→_____________ +___________
(b) Ca + H2O
→_____________ +___________
(c) Mg + H2O
→_____________ +___________
(d) Zn + H2O
→_____________ +___________
(e) Fe + H2O
→_____________ +___________
(f) Zn + HCl →_____________
+___________
(g) Al + H2SO4
→_____________ +___________
(h) Fe + HCl →_____________
+___________
(i) Zn + NaOH →_____________
+___________
(j) Al + KOH + H2O→_____________
+___________
Answer 12
(a) 2Na + 2H2O → 2NaOH +
H2
(b) Ca + 2H2O → Ca(OH)2
+ H2
(c) Mg + 2H2O → Mg(OH)2
+ H2
(d) Zn + H2O → ZnO + H2
(e) 3Fe + 4H2O
⇋Fe3O4
+ 4H2
(f) Zn + 2HCl → ZnCl2 +
H2
(g) 2Al + 3H2SO4
→Al2(SO4)3 + 3H2
(h) Fe +2HCl →FeCl2 + H2
(i) Zn + 2NaOH → Na2ZnO2
+ H2
(j) 2Al + 2KOH + 2H2O
→2KAlO2 + 3H2
Question 13
If the following are kept in closed
vessels at over 400°C, what would happen to them?
(a) iron filing and steam
(b) hydrogen and magnetic oxide of
iron?
Answer 13
(a) Iron oxide is formed with the
evolution of hydrogen gas.
(b) Hydrogen reduces heated
magnetic oxide of iron.
Question 14
(a) A metal in the powdered from
reacts very slowly with boiling water, but it decomposes in
steam. Name the metal.
(b) Write a balanced equation for
the reaction occurring in (a).
Answer 14
(a) Magnesium
(b)
(i) Mg + 2H2O → Mg(OH)2 + H2
(i) Mg + H2O →MgO + H2
Question 15
What do you observe when hydrogen
gas is passed through a soap solution?
Answer 15
On passing hydrogen gas through
soap solution, soap bubbles filled with hydrogen fly high
and burst. This behavior proves that hydrogen is lighter
than air.
Question 16
Under what conditions can hydrogen
be made to combine with?
(a) nitrogen?
(b) chlorine?
(c) sulphur?
(d) oxygen?
Name the products in each case and
write the equation for each reaction.
Answer 16
(a) Three volumes of hydrogen and
one volume of nitrogen react at temperature 450-500°C and
pressure 200-900 atm in the presence of finely divided iron
catalyst with molybdenum as promoter to give ammonia.
N2 + 3H2⇋
2NH3
(b) Equal volumes of hydrogen and
chlorine react slowly in diffused sunlight to form hydrogen
chloride.
H2
+ Cl2 →2HCl
(c) Hydrogen gas on passing through
molten sulphur reacts to give hydrogen sulphide.
H2
+ S → H2S
(d) Hydrogen burns in the presence
of electric spark with a ‘pop’ sound in oxygen and with a
blue flame forming water.
2H2 + O2 →2H2O
Question 17
When hydrogen is passed over a
black solid compound A, the products are a ‘colourless
liquid’ and a ‘reddish brown metal B’.
Substance B is divided into two
parts each placed in separate test tubes.
Dilute HCl is added to one part of
substance B and dilute HNO3 to the other.
(a) Name the substances A and B.
(b) Give two tests for the
colourless liquid formed in the experiment.
(c) What happens to substance A
when it reacts with hydrogen? Give reasons for your answer.
(d) Write an equation for the
reaction between hydrogen and substance A.
(e) Is there any reaction between
substance B and dilute hydrochloric acid? Give reasons for
your answer.
Answer 17
(a) A = CuO, B = Cu
(b) Blue and red litmus paper when
dipped in the colourless liquid do not change colour. This
confirms the liquid formed is neutral and is water.
It changes white anhydrous copper
sulphate to blue salt.
(c) Black copper oxide (A) on
heating with hydrogen reduces copper oxide to reddish brown
copper and itself gets oxidised to water.
Hydrogen is a strong reducing agent
and removes oxygen from less active metals, i.e. it removes
oxygen from heated metal oxides when passed over them and
itself gets oxidised to water.
(d) CuO + H2 Cu + H2O
Cu + HCl →No reaction
(e) Copper is less reactive than
hydrogen and hence cannot displace it from HCl.
Exercise – 6(D) Chapter-6
Question 1
Describe briefly the ionic concept of oxidation and
reduction. Give an equation to illustrate.
Answer 1
In the electronic concept, oxidation is a process in which
an atom or ion loses electron(s).
Zn → Zn2+ + 2e–
Oxidation is also defined
as a chemical process which involves
Addition of oxygen
Addition of electronegative ion
Removal of hydrogen
Removal of electropositive ion (element)
In the electronic concept, reduction is a process in which
an atom or ion gains electrons.
Cu2+ + 2e–→ Cu
Reduction is also defined
as a chemical process which involves
Removal of oxygen
Addition of electropositive ion
Addition of hydrogen
Removal of electronegative ion
Question 2
Is it essential that oxidation and reduction must occur side
by side in a chemical reaction? Explain
Answer 2
In a chemical reaction, if one substance is oxidised, the
other substance must necessarily be reduced. This is because
the electrons lost during oxidation are simultaneously
gained during reduction and vice versa.
For example: Zinc reacts with copper sulphate to form zinc
sulphate and copper.
CuSO4 + Zn →
ZnSO4 + Cu
Cu + 2SO42-
+ Zn →Zn + 2SO42- + Cu
Writing the half reaction,
Zn → Zn2+ + 2e–
(Oxidation)
Cu2+ + 2e–→
Cu (Reduction)
They occur simultaneously as
Cu2+ + Zn→ Zn2++
Cu
Thus, oxidation and reduction always occur simultaneously.
Question 3
State, giving reasons, whether the substances printed in
bold letters have been oxidized or reduced.
(a) PbO + CO → Pb + CO2
(b) Mg + 2HCl → MgCl2
+ H2
(c) H2S + Cl2→
2HCl + S
(d) Cl2 + H2S
→ 2HCl + S
Answer 3
(a) PbO in the given reaction is reduced to Pb by losing
oxygen.
(b) Magnesium undergoes oxidation by loss of electrons (Mg –
2e– → Mg2+).
(c) H2S undergoes oxidation by loss of hydrogen
to give sulphur.
(d) Chlorine undergoes reduction by the addition of hydrogen
to form HCl.
Question 4
State whether the following conversions are oxidation or
reduction:
(a) PbO2 + SO2→ PbSO4
(b) Cu2+ + 2 e–→ Cu
(c) K → K+ + e–
(d) 2Cl– – e–→ Cl2
Answer 4
(a) Oxidation
(b) Reduction
(c) Oxidation
(d) Oxidation
Question 5
In the following reaction: A+ + B → A + B+.
Write half reactions for this reaction and name:
(a) oxidizing agent
(b) substance oxidized
(c) reducing agent
Answer 5
Half reaction:
A+ + e–→ A (Reduction)
B → B + e– (Oxidation)
(a) A
(b) B
(c) B
Question 6
Divide the following reactions into oxidation and reduction
half reactions:
(i) Zn + Pb2+→
Pb + Zn 2+
(ii) Zn + Cu2+
→ Cu + Zn 2+
(iii) Cl2 + 2Br–
→ Br2 + 2Cl–
Answer 6
(i) Zn + Pb2+ →
Pb + Zn 2+
Zn → Zn 2+ + 2 e- —- Oxidation
Pb2+ + 2 e → Pb —- Reduction
(ii) Zn + Cu2+
→ Cu + Zn 2+
Zn → Zn 2+ + 2 e- —- Oxidation
Cu2+ + 2 e → Cu —- Reduction
(iii) Cl2 + 2Br–
→ Br2 + 2Cl–
Cl2→ 2Cl– + 2 e– —-
Oxidation
2Br‒ + 2 e → Br2—- Reduction
Question 7
(a) Write the equation in the ionic form
CuSO4(aq) + Fe(s)→
FeSO4(aq) + Cu(s)
(b) Divide the above equation into oxidation and reduction
half reactions.
Answer 7
(a) Equation in the ionic form:
Cu2+ SO42-
+ Fe → Fe2+ SO42- + Cu
(b) Fe → Fe2+ 2
e– —- Oxidation
Cu2+ + 2 e → Cu
—- Reduction
Question 8
Give reasons:
(a) Hydrogen is collected by the downward displacement of
water and not of air, even though it is lighter than air.
(b) A candle brought near the mouth of a jar containing
hydrogen gas starts burning but is extinguished when pushed
inside the jar.
(c) Apparatus for laboratory preparation of hydrogen should
be air tight and away from a naked flame.
Answer 8
(a) Hydrogen is collected by the downward displacement of
air because
i. It is insoluble in water.
ii. It forms an explosive mixture with air and therefore
cannot be collected by the downward displacement of air even
though it is lighter than it.
(b) Hydrogen is combustible, but it does not support
combustion. So, the candle burns in air or oxygen when
brought near the mouth of a jar containing hydrogen but is
extinguished when pushed inside the jar as the supply of
oxygen is cut off.
(c) Apparatus for laboratory preparation of hydrogen should
be airtight and away from a naked flame because a mixture of
hydrogen and air explodes violently when brought near a
flame.
Question 9
Select the odd one out and justify your answer.
(a) Zn, Fe, Mg and Na
(b) SO2, H2S, NH3 and CO3
(c) Fe, Zn, Cu and Mg
(d)
Fe, Pb, Al and Zn
Answer 9
(a) Na
The other metals react with dil. HCl liberating hydrogen
gas, while sodium reacts violently with acid.
(b) NH3 is basic in nature.
(c) Cu
Metals more reactive than hydrogen can displace it from
acids.
(d) Pb
Lead reacts with dilute sulphuric acid or HCl and forms an
insoluble coating of lead sulphate or lead chloride.
The others react with dilute sulphuric acid or HCl to
liberate hydrogen.
Question 10
(a) Helium is preferred to
hydrogen for filling balloons because it is:
(i) lighter than air
(ii) almost as light as hydrogen
(iii) non-combustible
(iv) inflammable
(b) Reacting with water,
an active metal produces
(i) oxygen
(ii) nitric acid
(iii) a base
(iv) none of these
(c) A metal oxide that is
reduced by hydrogen is
(i) Al2O3
(ii)CuO
(iii) CaO
(iv) Na2O
(d) Which of the following
statements about hydrogen is incorrect?
(i) It is an inflammable gas
(ii) It is the lightest gas.
(iii)It is not easily liquefied
(iv )It is a strong oxidizing agent.
(e) For the reaction PbO +
H2→ Pb + H2O, which of the following
statements is wrong?
(i) H2 is the reducing agent.
(ii) PbO is the oxidizing agent.
(iii) PbO is oxidized to Pb.
(iv) H2 is oxidized to H2O.
(f) Which metal gives
hydrogen with all of the following: water, acids, alkalis?
(i) Fe
(ii) Zn
(iii) Mg
(iv) Pb
(g) Which of the following
metals does not give hydrogen with acids?
(i) Iron
(ii) Copper
(iii) Lead
(iv) Zinc
Answer 10
(a) (iii) non-combustible
(b) (iii) base
(c) (ii) CuO
(d) (iv) It is a strong oxidising agent.
(e) (iii) PbO is oxidised to Pb.
(f) (ii) Zn
(g) (ii) Cu
Question 11
Choose terms from the options given in brackets to complete
these sentences.
(a) When CuO reacts with hydrogen,………………… is reduced and
……………….is oxidized to ………………… .
(CuO, H2,Cu,H2O)
(b )Hydrogen is
………………… soluble in water.
(sparingly, highly, moderately)
(c) Metals like
…………….. , ……………… and ……………… give H2 with steam.
(iron, magnesium, aluminium, sodium , calcium)
(d) Sodium ………………. reacts smoothly with cold water.
(metal, amalgam, in the molten state)
(e) A metal ……………..
hydrogen in the activity series gives hydrogen with ……………
acid or … ………… acid.
(above, below, dilute hydrochloric, concentrated
hydrochloric, dilute sulphuric).
Answer 11
(a) CuO, H2, H2O
(b) sparingly
(c) magnesium, iron and aluminium
(d) amalgam
(e) above, dilute hydrochloric, dilute sulphuric acid
Question 12
Correct the following statements:
(a) Hydrogen is separated from CO by passing the mixture
through caustic potash solution.
(b) All metals react with acids to give hydrogen.
(c) Hydrogen is dried by passing it through conc. H2SO4.
(d) Very dilute nitric acid reacts with iron to produce
hydrogen.
(e) Conc. H2SO4 reacts with zinc to
liberate hydrogen.
Answer 12
(a) Hydrogen is separated from CO by passing the mixture
through caustic potash solution.
(b) All metals above hydrogen in the activity series react
with acids to give hydrogen.
(c) Hydrogen is dried by passing it through calcium
chloride, caustic potash and phosphorous pentoxide.
(d) Very dilute nitric acid reacts with magnesium and
manganese to produce hydrogen.
(e) Dil. H2SO4 reacts with zinc to
liberate hydrogen.
Question 13
Name:
(a) an oxidizing agent that does not contain oxygen.
(b) a substance that oxidizes concentrated HCl to chlorine.
(c) a substance that will reduce aqueous Iron(III)ions to
Iron(II)ions.
(d) a liquid that is an oxidizing agent as well as a
reducing agent.
(e) a gas that is an oxidizing as well as a reducing agent.
(f) a solid that is an oxidizing agent.
Answer 13
(a) Chlorine
(b) MnO2
(c) H2S
(d) Hydrogen peroxide
(e) MnO2
Class 9 Chemistry Water
Question
1
Water exists in all three states.
Discuss.
Answer 1
In the Free State, water occurs in
the solid, liquid and gaseous states.
(a)Solid state: A large amount of
fresh water is found in the form of snow or ice.
(b)Liquid state: Most of the water
present in oceans and found in streams, rivers, lakes, ponds
and springs on land is water in the liquid state.
(c)Gaseous state: Water vapour
present in the air is in the gaseous state. Water vapour
condenses in the sky to form clouds. Mist and fog are also
examples of water in the gaseous form.
Question 2
Why water is considered a compound?
Answer 2
Water is considered a compound
because it is made of two elements hydrogen and oxygen
combined in the ratio 1:8 by mass.
Components of water cannot be
separated by physical methods but can be separated by
electrolysis of water.
Question 3
(a)Why does temperature in Mumbai
and Chennai not fall as low as it does in Delhi?
(b)Give the properties of water
responsible for controlling the temperature of our body.
Answer 3
a)The temperature in Mumbai and
Chennai do not fall as low as in Delhi because these cities
are situated in the coastal areas. Due to high specific heat
capacity, the presence of a large amount of water is able to
modify the climate of the nearby land areas making them
warmer in winter and cooler in summer. So, the temperature
does not fall as low as it does in Delhi.
b) Our body is almost 65% of water,
and it has the property of specific heat. Due to high
specific heat capacity, the presence of a large amount of
water is able to modify the climate of the body and control
the temperature of our body, which is warm in winter and
cool in summer.
Question 4
‘Water is the universal solvent’.
Comment.
Answer 4
Water dissolves many substances
forming an aqueous Solution. It can dissolve solids, liquids
and gases. When a solid dissolves in water, the solid is the
solute, the water is the solvent and the resultant liquid is
the Solution. So, it is said that water is a universal
solvent. In other words, water can dissolve nearly every
substance.
Question 5
What causes the violence associated
with torrential rain?
Answer 5
The sudden release of the latent
heat of condensation causes the violence associated with
torrential rain.
Question 6
(a)Which property of water enables
it to modify the climate?
(b)Density of water varies with
temperature. What are its consequences?
(c)What is the effect of impurities
present in the water on the melting point and boiling point
of water?
Answer 6
(a)Due to the high specific heat
capacity, the presence of a large amount of water is able to
modify the climate.
(b)The property of anomalous
expansion of water enables marine life to exist in the
colder regions of the world, because even when water freezes
on the top, it is still liquid below the ice layer, as the
density of water is greater than that of ice.
(c)The boiling point of water
increases due to the presence of dissolved impurities.
The freezing point of water
decreases due to the presence of dissolved impurities.
Question 7
How do fishes and aquatic animals
survive when the pond gets covered with thick ice?
Answer 7
Water has an unusual physical
property. When cooled, it first contracts in volume, as do
other liquids, but at 4°C (maximum density), it starts
expanding, and continues to do so till the temperature
reaches 0°C, the point at which it freezes into ice.
Question 8
The properties of water are
different from the properties of the elements of which it is
formed. Discuss.
Answer 8
|
Properties of water are different from the
properties of elements from which it is formed.
|
|
Property
|
Water
|
Elements – Oxygen and Hydrogen
|
|
Nature
|
It is a clear, colourless,
odourless, tasteless and transparent liquid.
|
These are colourless,
odourless, tasteless and non-poisonous gases.
|
|
Solubility
|
It can dissolve many
substances and is called a universal solvent.
|
Oxygen and hydrogen are
slightly soluble in water.
|
|
Density
|
Pure water has maximum
density at 4°C.
|
Oxygen is heavier than air,
and hydrogen is the lightest of all the known gases.
|
|
|
|
|
Question 9
How is aquatic life benefited by
the fact that water has maximum density at 4oC?
Answer 9
The property of anomalous expansion
of water enables aquatic life to exist because water freezes
on the surface of the water body, but it is still liquid
below the ice layer.
Question 10
What are the observations and
conclusions when tap water is boiled and evaporated in a
water glass?
Answer 10
When tap water is boiled and
evaporated:
Observations:
(a) A number of concentric rings of
solid matter are seen on the watch glass after evaporation
of tap water.
Conclusion:
(b) Tap water contains dissolved
salts, minerals and impurities.
Question 11
What is the importance of dissolved
salts in water?
Answer 11
Importance of dissolved salts in
water:
(a)Dissolved salts provide specific
taste to water.
(b)Dissolved salts act as
micronutrients for the growth and development of living
beings.
Question 12
State the importance of the
suitability of CO2 and O2 in water.
Answer 12
They add taste to water for
drinking purposes.
Question 13
How is air dissolved in water
different from ordinary air?
Answer 13
Oxygen is more soluble in water than nitrogen. Air dissolved
in water contains a higher percentage of oxygen (30-35%).
Oxygen is only 21% in ordinary air. In this way, air
dissolved in water is different from ordinary air.
Question 14
Identify A, B, C, and D first one is done for you.
Answer 14
When a solid changes into a liquid, it absorbs heat equal to
the latent heat of fusion. When a liquid changes into a
solid, it loses heat equal to the latent heat of
solidification.
a liquid changes into a gas, it absorbs heat equal to the
latent heat of vaporisation. When a gas condenses into a
liquid, it loses heat equal to the latent heat of
condensation.
Question 15
Explain why:
(a)Boiled or distilled water tastes flat.
(b)Ice at zero degrees centigrade has greater cooling effect
than water at 0oC.
(c)Burns caused by steam are more severe than burns caused
by boiling water.
(d)Rivers and lakes do not freeze easily?
(e)Air dissolved in water contains a higher proportion of
oxygen.
(f)If distilled water is kept in a sealed bottle for a long
time, it leaves etchings on the surface of the glass.
(g)Rain water does not leave behind concentric rings when
boiled.
Answer 15
(a)Boiled water tastes flat because it does not contain
dissolved matter such as air, carbon dioxide and other
minerals.
(b)Ice at 0°C gives more cooling effect than water at 0°C
because at 0°C ice absorbs 336 J per gram of energy to melt
to 0°C water.
(c)Burns caused by steam are more severe than burns caused
by boiling water because of high specific latent heat of
condensation. 2268 J/g of heat is released when 1 g of steam
condenses to form 1 gm of water.
(d)Due to the high specific latent heat of solidification of
water, rivers and lakes do not freeze easily.
(e)Air dissolved in water contains a higher percentage of
oxygen because the solubility of oxygen in water is more
than that of oxygen in air.
(f)If distilled water is kept in a sealed bottle for a long
time, it etches the surface of glass because substances
which are apparently insoluble in water actually dissolve in
minute traces in water.
(g)Rain water does not leave concentric rings when boiled
because rain water does not contain dissolved solids.
Exercise – 3(B)
Question 1
Explain the terms:
(Answer
1
(a)Solution:
A Solution is a homogeneous mixture of two or
more substances, the components of which cannot be seen
separately.
(b)Solute:
A solute is the substance which dissolves in a solvent
to form a Solution.
(c)Solvent: A solvent is the medium in which a solute
dissolves.
|
Solution = Solute + Solvent
|
Question 2
Explain why a hot saturated Solution of potassium nitrate
forms crystals as it cools.
Answer 2
Solubility of nitrates decreases with a fall in temperature.
Thus, when a hot saturated Solution of potassium nitrate
cools, it forms crystals as it separates from the Solution.
Question 3
Give three factors which affect the solubility of a solid
solute in a solvent.
Answer 3
Three factors on which the solubility of a solid depend:
(i)Temperature
(ii)Nature of the solid
(iii)Nature of the solvent
Question 4
(a) If you are given some copper sulphate crystals, how
would you proceed to prepare its saturated Solution at room
temperature?
(b) How can you show that your Solution is really saturated?
Answer 4
(a)Take 100 g of distilled water in a beaker. Add to this
one gram of copper sulphate crystals.
(b) This mixture with the help of a glass rod and dissolve
the copper sulphate crystals. Similarly, go on dissolving
more copper sulphate (1 gram at a time) with constant and
vigorous stirring. A stage is reached when no more copper
sulphate dissolves. It is called a saturated Solution at
this temperature.
Question 5
(a)Define (i) Henry’s law and (ii) Crystallisation (iii)
Seeding.
(b)State the different methods of crystallisation.
Answer 5
(a)
(I) Henry’s law:
At any given temperature, the mass of a gas dissolved in a
fixed volume of a liquid or Solution is directly
proportional to the pressure on the surface of a liquid.
(ii) Crystallisation:
It is the process by which crystals of a substance separate
out on cooling its hot saturated Solution.
(iii) Seeding:
This process of inducting crystallisation by adding a
crystal of pure substance into saturated solution is called
seeding.
(b)
In the laboratory, crystals may be obtained by the following
methods:
(i)By cooling a hot saturated Solution gently
(ii)By cooling a fused mass
(iii)By sublimation
(iv)By slowly evaporating a saturated Solution
Question 6
What would you observe when crystals of copper (II) sulphate
and iron (II) sulphate are separately heated in two test
tubes strongly?
Answer 6
Action of heat on copper (II) sulphate crystals
When copper (II) sulphate crystals are heated in a hard
glass test tube, the following observations are observed:
(i)The crystals are converted to a powdery substance.
(ii)The crystals lose their blue coloration on further
heating.
(iii)Steaming vapors are produced inside the tube which
condense near the mouth of the tube to form a colorless
liquid.
(iv)On further heating, steam escapes from the mouth of the
tube and water gets collected in a beaker placed under the
mouth of the tube.
(v)On further heating, the residue changes to a white powder
and steam stops coming out.
|
CuSO4.5H2O → CuSO4
+ 5H2O
|
Action of heat on iron
(II) sulphate
When iron (II) sulphate is heated in a test tube, the
following is observed:
(i)The crystals crumble to a white powder and a large amount
of steam and gas are given out.
(ii)On strong heating, a brown residue of ferric oxide (Fe2O3)
is produced and a mixture of SO2 and SO3
is given off.
Question 7
Give the names and formulae of two substances in each case:
(a)Hydrated substance
(b)Anhydrous substance
(c)Liquid drying agent
(d)A basic drying agent
Answer 7
(a) (i) Washing soda crystals: Na2CO3.10H2O
(ii) Blue vitriol: CuSO4.5 H2O
(b) (i) Table salt:
NaCl
(ii) Nitre: KNO3
(c)Sulphuric acid: H2SO4
(d)Quick lime: CaO
Question 8
What is the effect of temperature on solubility of KNO3
and CaSO4 in water?
Answer 8
Solubility of potassium nitrate (KNO3) in water
increases with an increase in temperature.
Solubility of calcium sulphate (CaSO4) in water
decreases with an increase in temperature.
Question 9
Solubility of NaCl at 40oC is 36.5 g. What is
meant by this statement?
Answer 9
Solubility of NaCl at 40°C is 36.5 g means that 36.5 g of
NaCl dissolves in 100 g of water at a temperature of 40°C.
Question 10
Which test will you carry out to find out if a given
Solution is saturated or unsaturated or supersaturated?
Answer 10
A Solution in which more of a solute can be dissolved at a
given temperature is an unsaturated Solution.
The Solution in which no more solute can be dissolved at a
given temperature is a saturated Solution at that
temperature.
Solution in which some solute separates on cooling slightly
is a super saturated Solution.
Question 11
What is the effect of pressure on solubility of gases?
Explain with an example.
Answer 11
With an increase in pressure, the solubility of a gas in
water increases.
With an increase in temperature, the solubility of a gas in
water decreases.
For example, the solubility of carbon dioxide in water under
normal atmospheric pressure is low, but when the water
surface is subjected to higher pressure, a lot more of CO2
gas gets dissolved in it.
Similarly, in case of soda water, on opening the bottle, the
dissolved gas rapidly bubbles out because the pressure on
the surface of the water suddenly decreases.
Question 12
State the term:
(a)A Solution where solvent is a liquid other than water.
(b)When a substance absorbs moisture on exposure to moist
air and dissolves in the absorbed water and turned to
Solution.
(c)A substance which contains water of crystallisation.
(d)When a substance absorbs moisture from the atmosphere but
does not form a Solution.
(e)When a compound loses its water of crystallisation on
exposure to dry air.
(f)The substance that can remove hydrogen and oxygen atoms
in the ratio of 2:1(in the form of water) from the compound.
Answer 12 Water
(a)Non-aqueous Solution
(b)Deliquescence
(c)Hydrated substance
(d)Hygroscope
(e)Efflorescence
(f)Dehydrating agent
Question 13
Explain why:
(a)Water is an excellent liquid to use in cooling systems.
(b)A Solution is always clear and transparent.
(c)Lakes and rivers do not suddenly freeze in the winters.
(d)The solute cannot be separated from a Solution by
filtration.
(e)Fused CaCl2 or conc. H2SO4
is used in a desiccator.
(f)Effervescence is seen on opening a bottle of soda water.
(g)Table salts become sticky on exposure to humid air during
the rainy season.
Answer 13
(a)Water is an excellent liquid to use in cooling systems
because of its high specific heat.
(b)A water-soluble solid disappears in a Solution where the
solvent is water, and water has the property of being clear
and transparent. So, the Solution is also clear and
transparent.
(c) Lakes and rivers do
not freeze suddenly in winters
because of the high specific latent heat of solidification,
i.e. the amount of heat released when 1 g of water
solidifies to form 1 g of ice at 0°C. It is about 336 J/g or
80 cal/g.
(d)The component which dissolves in a solvent is known as a
solute. So, it cannot be separated from a Solution by
filtration. However, filtration is used when the solute is
insoluble in the Solution.
(e)Fused CaCl2 or concentrated H2SO4
is deliquescent in nature, i.e. it absorbs moisture, and
hence, these are used in desiccators as drying agents.
(f)Carbon dioxide is dissolved in soda water under pressure.
On opening the bottle, the pressure on the surface of water
suddenly decreases; therefore, the solubility of CO2
in water decreases and the gas rapidly bubbles out.
(g)Table salt becomes sticky on exposure during the rainy
season, because it generally contains a small percentage of
magnesium chloride and calcium chloride as impurities. These
impurities absorb moisture from the monsoon air due to their
deliquescent nature, and thus, table salt become sticky.
Question 14 Water
Name a substance whose solubility:
(a)Increases rapidly with temperature.
(b) solubility Increases gradually with temperature.
(c) Increases slightly with temperature.
(d) Initially increases then decreases with rise in
temperature.
Answer 14
(a)Potassium nitrate
(b)Potassium chloride
(c)Sodium chloride
(d)Calcium sulphate
Question 15
What are drying or desiccating agents? Give examples.
Answer 15
These are substances which can readily absorb moisture from
other substances without chemically reacting with them.
Examples:
Phosphorous pentoxide (P2O5), quick
lime (CaO)
Answer 16
|
Common Name
|
Chemical Name
|
Formula
|
Acid, base or salt
|
Efflorescent,
hygroscopic or deliquescent substance
|
|
Solid caustic potash
|
Potassium hydroxide
|
KOH
|
Base
|
Deliquescent substance
|
|
Quick lime
|
Calcium oxide
|
CaO
|
Base
|
Hygroscopic substance
|
|
Oil of vitriol
|
Sulphuric acid
|
H2SO4
|
Acid
|
Hygroscopic substance
|
|
Washing soda
|
Hydrated sodium carbonate
|
Na2CO3.10H2O
|
Salt
|
Efflorescent substance
|
|
Solid caustic soda
|
Sodium hydroxide
|
NaOH
|
Base
|
Deliquescent substance
|
|
Blue vitriol
|
Copper sulphate
|
CuSO4
|
Salt
|
Efflorescent substance
|
Question 17 Water Selina
Solutions Chapter-3
In which of the following substances will there be
(a)Increase in mass
(b)Decrease in mass
(c)No change in mass when they are exposed to air?
Sodium chloride
Iron
Conc. sulphuric acid
Table salt
Sodium carbonate crystals
Answer 17
(a)Increase in mass: Iron and conc. sulphuric acid
(b)Decrease in mass: Sodium carbonate crystals
(c)No change in mass: Sodium chloride
Question 18
State the methods by which hydrated salts can be made
anhydrous.
Answer 18
Hydrated salts can be converted to anhydrous substances by
heating and also when exposed to dry air.
Example:
Gaber’s salt becomes powdery anhydrous sodium sulphate when
exposed to dry air.
Exercise – 3(C
Question 1
What is the composition of water? In what volume its
elements combine?
Answer 1
The composition of water is 2 atoms of hydrogen with 1 atom
of oxygen (H2O).
By number of atoms, they combine in the ratio 2:1.
Question 2
What is the use of solubility of oxygen and carbon dioxide
in water?
Answer 2 Water Selina
Solutions Chapter-3
Air dissolved in water is biologically very important.
Oxygen dissolved in water is used by marine life like fish
for respiration, and thus, marine life is sustained.
Aquatic plants make use of dissolved carbon dioxide in
photosynthesis to prepare food.
Carbon dioxide dissolved in water reacts with calcium
carbonate to form calcium bicarbonate.
Marine organisms such as oysters and snails extract calcium
carbonate from calcium bicarbonate to build their shells.
Question 3
Hot saturated Solution of sodium nitrate forms crystals as
it cools. Why?
Answer 3
Solubility of sodium nitrate decreases with a fall in
temperature. Thus, when a hot saturated Solution of sodium
nitrate cools, it forms crystals as it separates from the
Solution.
Question 4
What are hydrous substances? Explain with examples.
Answer 4
Substances which contain water molecules along with salt are
hydrated substances.
Examples: Sodium carbonate dehydrate: Na2CO3.10H2O
Copper sulphate pentahydrate: CuSO4.5H2O
Question 5
Name three methods [CM1] by which hydrous substances can be
made anhydrous.
Answer 5
Methods by which hydrous substances can be made anhydrous:
By heating and by Exposure to dry air
Question 6
What is the importance of dissolved impurities in water?
Answer 6
The dissolved impurities in water are salts and minerals.
Dissolved salts provide specific taste to water.
Salts and minerals are essential for growth and development.
They supply the essential minerals needed by our body.
Question 7 Water
State two ways by which a saturated Solution can be changed
to unsaturated Solution.
Answer 7
On heating, a saturated Solution becomes unsaturated and
more solute can be dissolved in the Solution.
By adding more solvent, a saturated Solution can be made
unsaturated.
Question 8
What do you understand by?
(a)Soft water
(b)Hard water
(c)Temporary hard water
(d)Permanent hard water
Answer 8
(a)Water is said to be soft when the water containing sodium
salts easily gives lather with soap.
(b)Water is said to be hard when it does not readily form
lather with soap.
(c)Water which contains only hydrogen carbonates of calcium
and magnesium is called temporary hard water.
(d)Water containing sulphates and chlorides of magnesium and
calcium is called permanent hard water.
Question 9
What are the causes for?
(a)Temporary hardness
(b)Permanent hardness
Answer 9
(a)The presence of hydrogen carbonates of calcium and
magnesium makes water temporarily hard.
(b)The presence of sulphates and chlorides of magnesium and
calcium makes water permanently hard.
Question 10 Water
What are the advantages of (i) soft water and (ii) hard
water?
Answer 10
Advantages of soft water:
1) When the water is soft, you use much less soap and fewer
cleaning products. Your budget will reflect your savings.
2)Plumbing will last longer. Soft water is low in mineral
content and therefore does not leave deposits in the pipes.
3)Clothes last longer and remain bright longer if they are
washed in soft water.
Advantages of hard water:
1) Water free from dissolved salts has a very flat taste.
The presence of salts in hard water makes it tasty. So, hard
water is used in making beverages and wines.
2) Calcium and magnesium salts present in small amounts in
hard water are essential for bone and teeth development.
3) Hard water checks the poisoning of water by lead pipes.
When these pipes are used for carrying water, some lead
salts dissolve in water to make it poisonous. Calcium
sulphate present in hard water forms insoluble lead sulphate
in the form of a layer inside the lead pipe and this checks
lead poisoning.
Question 11
What are stalagmites and stalactites? How are they formed?
Answer 11
In some limestone caves, conical pillar-like objects hang
from the roof and some rise from the floor. These are formed
by water containing dissolved calcium hydrogen carbonate
continuously dropping from the cracks in the rocks. Release
of pressure results in the conversion of some hydrogen
carbonate to calcium carbonate.
Ca (HCO3)2
→ CaCO3 + CO2 + H2O
This calcium carbonate little by little and slowly deposit
on both roof and floor of the cave.
The conical pillar which grows downwards from the roof is
called stalactite and the one which grows upward from the
floor of the cave is called stalagmite.
These meet after a time. In a year, some grow less than even
a centimetre, but some may be as tall as 100 cm.
CaCO3 + CO2
+ H2O → Ca (HCO3)2
MgCO3 + CO2
+ H2O → Mg (HCO3)2
If the water flows over beds of gypsum (CaSO4.2H2O),
a little bit of gypsum gets dissolved in water and makes it
hard.
Question 12 Water Selina
Solutions Chapter-3
Name the substance which makes water (i) temporarily hard
and (ii) permanently hard.
Answer 12
(i)Hydrogen carbonates of calcium and magnesium
(ii)Sulphates and chlorides of magnesium and calcium
Question 13
Give equations to show what happens when temporary hard
water is
(a)Boiled
(b)Treated with slaked lime
Answer 13
(a) when boiled
Ca (HCO3)2
→ CaCO3 + H2O + CO2↑
Mg (HCO3)2
→ MgCO3 + H2O + CO2↑
(b) when Treated with slaked lime
Ca(HCO3)2+
Ca(OH)2 → 2CaCO3 + 2H2O
Mg (HCO3)2+
Ca(OH)2→ MgCO3 + 2H2O
Question 14
State the disadvantages of using hard water.
Answer 14 disadvantages of
using hard water.
It is more difficult to form lather with soap.
Scum may form in a reaction with soap, wasting the soap.
Carbonates of calcium and magnesium form inside kettles.
This wastes energy whenever you boil a kettle.
Hot water pipes ‘fur up’. Carbonates of calcium and
magnesium start to coat the inside of pipes which can
eventually get blocked.
Question 15
What is soap? For what is it used?
Answer 15
Soap is chemically a sodium salt of stearic acid (an organic
acid with the formula C17H35COOH) and
has the formula C17H35COONa.
Soap is used for washing purposes.
Question 16
What is the advantage of a detergent over soap?
Answer 16
Detergents are more soluble in water than soap and are
unaffected by the hardness of water as their calcium salts
are soluble in water.
Question 17
Why does the hardness of water render it unfit for use in a
(i) boiler and (ii) for washing purposes.
Answer 17
Steam is usually made in boilers which are made of a number
of narrow copper tubes surrounded by fire. As the cold water
enters these tubes, it is immediately changed into steam,
while the dissolved solids incapable of changing into vapour
deposit on the inner walls of the tubes.
This goes on and makes the bore of the tubes narrower. The
result is that less water flows through the tubes at one
time and less steam is produced. When the bore of the tube
becomes very narrow, the pressure of the steam increases so
much that at times the boiler bursts.
If hard water is used,
calcium and magnesium ions of the water combine with the
negative ions of the soap to form a slimy precipitate of
insoluble calcium and magnesium usually called soap curd
(scum).
Formation of soap curd will go on as long as calcium and
magnesium ions are present. Till then, no soap lather will
be formed and cleaning of clothes or body will not be
possible. Moreover, these precipitates are difficult to wash
from fabrics and sometimes form rusty spots if iron salts
are present in water.
Question 18
Explain with equation, what is noticed when permanent hard
water is treated with
(a)Slaked time
(b)Washing soda
Answer 18
(a) Slaked lime
Ca (HCO3)2
+ Ca(OH)2 2CaCO3 + 2H2O
Mg (HCO3)2+
Ca(OH)2 MgCO3 + CaCO3 + 2H2O
Lime is first thoroughly mixed with water in a tank and then
fed into another tank containing hard water. Revolving
paddles thoroughly mix the two Solutions. Most of the
calcium carbonate settles down. If there is any solid left
over, it is removed by a filter. This is known as Clarke’s
process.
(b) Washing soda
When washing soda or soda ash is added to hard water, the
corresponding insoluble carbonates settle down and can be
removed by filtration.
Ca (HCO3)2
+ Na2CO3 CaCO3 + 2NaHCO3
Mg (HCO3)2+
Na2CO3 MgCO3 + 2NaHCO3
Question 19
Explain the permit method, how can it be used for softening
hard water.
Answer 19
Permit is an artificial zeolite. Chemically, it is hydrated
sodium aluminium or tho silicate with the formula Na2Al2Si2O8.XH2O.
For the sake of convenience, let us give it the formula Na2P.
A tall cylinder is loosely fille with lumps of permit. When
hard water containing calcium and magnesium ions percolates
through these lumps, ions exchange. Sodium permit is slowly
changed into calcium and magnesium permit, and the water
becomes soft with the removal of calcium and magnesium ions.
When no longer active, permit is regenerated by running a
concentrated Solution of brine over it and removing calcium
chloride formed by repeated washing.
CaP + 2NaCl → Na2P
+ CaI2
Chemical Changes and Reactions
Question 1
(a)
What is a chemical reaction?
(b)
State the conditions necessary for a chemical change or
reaction.
Answer
1
(a)
A
chemical reaction is the process of breaking the chemical
bonds of the reacting substances (reactants) and making new
bonds to form new substances (products).
(b)
Conditions necessary for a chemical change or reaction are
(i)
Evolution of gas
(ii)
Change of colour
(iii)
Formation of precipitate
(iv)Change of state
Question 2
Define
the following terms
(a). Chemical bond
(b). Effervescence
(c). Precipitate
Answer
2
(a). A
chemical bond is the force which holds the atoms of a
molecule together as in a
compound.
(b). Formation of gas bubbles in a
liquid during a reaction is called effervescence.
(c). Chemical reactions which are
characterised by the formation of insoluble solid substances
are called precipitates.
Question 3
Give an
example of a reaction where the following are involved
(a)
Heat
(b)
Light
(c)
Electricity
(d)
Close contact
(e)
Solution
(f)
Pressure
(g)
Catalyst
Answer
3
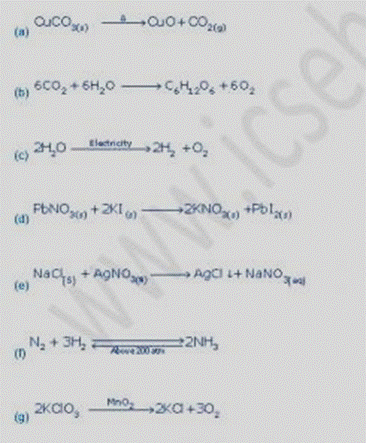
Question 4
Define
(a) Photochemical reaction (b)
Electrochemical reaction Give an example in each case.
Answer4
(a)
It
is a reaction which occurs with absorption of light energy.
(b)
It
is a reaction which occurs with absorption of electrical
energy.
Question 5
Give an
example of each of the following chemical changes:
(a) A photochemical reaction
involving
(i) Silver salt (ii) water
(b)A reaction involving
(i)
Blue Answer
(ii)
Formation of dirty green precipitate
(c)Two gases combine to form white
solid
(d)Two solids combine to form a
liquid
(e)A reaction where colour change is
noticed
REFER THE BOOK CHAPTER OF NOTES
PROVIDED
Question 6
Write the
chemical reaction where the following changes are observed.
(a)
Gas is evolved
(b)
Colour change is noticed (c) Precipitate is formed
(d) Physical state is changed
Answer 6 REFER
THE BOOK CHAPTER OF NOTES PROVIDED
Question 7
Give
reason for the following:
(a)
Silver nitrate Answer is kept in coloured bottles.
(b)
Molybdenum is used in the manufacture of ammonia.
(c)
Blue Answer of copper sulphate changes to green when a piece
of iron is added to this
solution.
(d)
Colourless concentrated sulphuric acid in a test tube
changes to blue on adding a small piece of copper to it.
Answer
7
(a)Silver
nitrate Answer is kept in brown bottles in the laboratory
because it decomposes in the presence of light.
(b)Molybdenum increases the
efficiency of the catalyst iron used in the manufacture of
ammonia.
(c)This is because the blue colour
of the copper sulphate Answer fades and eventually turns
into light green due to the formation of ferrous sulphate.
(d)Copper displaces hydrogen from
sulphuric acid and forms blue-coloured copper sulphate and
hydrogen gas is evolved.
Exercise-2(B), Chapter-2
Question 1
Complete
the following statements:
(a)The chemical change involving
iron and hydrochloric acid illustrates a _________________
reaction.
(b)In the type of reaction
called_______________, two compounds exchange their positive
and negative radicals.
(c)A catalyst either ______ or
_____________ the rate of a chemical change but itself
remains ______________ at the end Of the reaction.
(d)On heating, hydrated copper
sulphate changes its colour from ________ to __________.
Answer
1
(a)Displacement
(b)Double decomposition
(c)Accelerates, decelerates,
unaffected
(d)Blue, white
Question 2
When hydrogen burns in oxygen,
water is formed; when electricity is passed through water,
hydrogen and oxygen are given out. Name the type of chemical
change involved in the two cases.
Answer
2
When
hydrogen burns in oxygen, water is formed – Combination
Reaction.
When electricity is passed through
water, hydrogen and oxygen are given out – Decomposition
Reaction.
Question 3
Explain,
giving one example for each of the following chemical
changes:
(a)
Double decomposition
(b)
Thermal decomposition
(c)
Reversible reaction
(d)
Displacement
Answer 3
(a)
Double decomposition reaction
This is a type of chemical change
in which two compounds in solution to form two new compounds
by mutual exchange of radicals
(b)
Thermal decomposition
A
decomposition reaction brought about by heat is known as
thermal decomposition.
2HgO(s) 2Hg(s)
+O2(g)
(c) Reversible reaction
A
chemical reaction in which the direction of a chemical
change can be reversed by changing the conditions under
which the reaction is taking place is called a reversible
reaction.
CuSO4.5H2O(s)
 CuSO4(s)
+ 5H2O
(g) CuSO4(s)
+ 5H2O
(g)
(d) Displacement Reaction
It is a
chemical change in which a more active element displaces a
less active element from its salt solution.
CuSO4 + Zn → ZnSO4
+ Cu
Question 4
(a)
What is synthesis?
(b)
What kind of chemical reaction is synthesis? Support your
answer by an example.
Answer
4
A
reaction in which two or more substances combine together to
form a single substance is called a synthesis or combination
reaction.
A + B → AB
In the above reaction, substances A
and B combine to give a molecule of a new substance, AB.
Carbon burns in oxygen to form a gaseous compound, carbon
dioxide.
C + O2
== CO2
Question 5
Decomposition brought about by heat
is known as thermal decomposition. What is the difference
between thermal dissociation and thermal decomposition?
Answer
5
A
decomposition reaction brought about by heat is known as
thermal decomposition.
2HgO(s) —- 2Hg(s) + O2
(g)
A simultaneous reversible
decomposition reaction brought about only by heat is thermal
dissociation.
NH4Cl
 NH3
+HCl NH3
+HCl
Question 6
(a)Define
neutralization reaction with an example.
(b)Give
balanced equation for this reaction.
(c)Give three applications of
neutralization reactions.
Answer
6
(a)The
reaction between an acid and a base to form salt and water
only is referred to as a neutralisation reaction.
(b)NaOH + HCl → NaCl + H2O
(c) Applications of neutralisation reactions:
(i) When
someone is stung by a bee, formic acid enters the skin and
causes pain, which can be relieved by rubbing the spot with
slaked lime or baking soda, both of which are bases. (ii)
Acid which is accidentally spilled on to our clothes can be
neutralised with ammonia solution.
(iii) If soil is somewhat acidic and
thus unfavourable for growing of certain crops, slaked lime
is added to neutralise the excess acid.
Question 7
What do you understand by
precipitation reaction? Explain with an example.
Answer
7
A
chemical reaction in which two compound in their aqueous
state react to form an insoluble salt (a precipitate) as one
of the product is known as precipitation reaction.
For example: BaCl2 (aq)
+NaSO4 (aq) →BaSO4(s) white ppt +
2NaCl (aq).
Question 8
(a)What
are double displacement reactions?
(b) Give an example of double
displacement reaction, where a gas is evolved.
Answer
8
(a)This
is a type of chemical change in which two compound in a
solution react to form two compound by mutual exchange of
radicals Double decomposition reaction is also called double
displacement reaction.
AB +CD→ AD +CB
(b) Double displacement reaction,
gas evolved are
FeS(s) + H2SO4
(aq) → FeSO4 (aq) + H2S
Question 9
(a)What
is a decomposition reaction?
(b) Decomposition reaction can occur
by (i) heat
(ii) Electricity and (iii) sunlight
Give two balanced reaction for
reaction
Answer
9
(a)The
chemical reaction in which a compound splits into two or
more simpler substance (elements or compound) is called
decomposition reaction. 1 2HgO(s) —- 2Hg(s) + O2
(g)
2)DECOMPOSITION OF WATER BY PASSING
CURRENT 3)
DECOMPOSITION OF
SILVER NITRATE
Question 10
State the type of reactions each of
the following
Answer 10
(a)
Cl2
+ 2KBr → 2KCl + Br2
Displacement
reaction
(b)
NaOH + HCl → NaCl + H2O
Neutralisation reaction
(c)
2HgO → 2Hg + O2
Decomposition
reaction
(d)
Fe
+ CuSO4 → FeSO4 + Cu
Displacement reaction
(e)
PbO2 + SO2 → PbSO4
Combination reaction
(f)
2KClO3 → 2KCl + 3O2
Decomposition reaction
(g)
2H2O2 → 2H2O + O2
Decomposition reaction
(h)
KNO3 + H2SO4→ HNO3
+ KHSO4
Double decomposition reaction
(i)
CuO+H2 → Cu+ H2O
Displacement reaction
(j)
CaCO3 → CaO+ CO2
Decomposition reaction
(k)
NH4Cl
→ NH3 + HCl
Decomposition reaction
(l)
PbO + 2HNO3 → Pb(NO3) + 2H2O
Neutralisation reaction
(m)
AgNO3 + NaCl → AgCl + NaNO3
Double decomposition reaction
Question 12
Multiple choice
(a) Which of the following is not a
characteristic of a chemical change?
(i)
It
is irreversible.
(ii)
No
net energy change is involved.
(iii). New substance is formed.
(iv)Involves absorption or
liberation of energy.
(b) A reaction of a
type: AB + CD → AD + CD, involves
(i)
No
chemical change
(ii)
Decomposition of AB and CD
(iii)
.
Exchange of ions of AB and CD
(iv)
Combination of AB and CD
(c)
The reaction BaCl2(aq)+ H2SO4(aq)
→ BaSO4(s) + 2HCl(aq) is
(i)
Displacement reaction
(ii)
Neutralisation reaction
(iii)
.
Decomposition reaction
(iv)
Double displacement reaction
(d) Thermal
decomposition of sodium carbonate will produce
(i)
Carbon dioxide
(ii)
Oxygen
(iii)
Sodium hydroxide
(iv)
No
other product
Answer
12
(a)
(ii) No net energy change is involved
(b)
(iii) Exchange of ions of AB and CD
(c)
(iv)double displacement reaction
(d)
(i) Carbon dioxide
Exercise -2 (C),
Question 1
What is a chemical change? Give two
examples of chemical change?
Answer
1
A
chemical change is a permanent change in which the chemical
composition of a substance is changed and a new substance is
formed.
Examples:
Heating of copper carbonate
Formation of curd from milk
Question 2
Why energy is involved in a chemical
change?
Answer
2
In every
chemical change, change in energy is involved.
There is a difference between the
chemical energies of the reactants and products. It involves
the breaking up of chemical bonds between the atoms
resulting in the absorption of energy in the form of heat
and simultaneous formation of bonds with the release of
energy. Question 3
What do you understand by ‘chemical
reaction’?
Answer
3
A
chemical reaction is the process of breaking the chemical
bonds of the reacting substances (reactants) and making new
bonds to form new substances (products).
A chemical change or chemical
reaction occurs when particles collide. Collisions occur
when reactants are in close contact or by supply of energy.
Question 4
Give an
example of a reaction where the following are involved
(a)
Evolution of heat
(b)
Absorption of heat
(c)
High pressure is required
Answer 4
(a)
C
+ O2 → CO2 + Heat
(b)
C
+ 2S → CS2
(c)
N2
+ 3H2→
2NH3
Question 5
State the main characteristics of
chemical reactions. Give at least one example in each case.
Answer
5
(i)
Evolution of gas Example:
Zn + H2SO4 →
ZnSO4 + H2
[Zinc] + [dil.sulphuricAcid] [zinc
sulphate] [hydrogen]
(ii)
Change of colour Example:
Fe + CuSO4 (aq) → FeSO4 +
Cu
[Iron] [Blue solution] [Green
Solution] [copper]
(iii) Formation of precipitates: Example:
AgNO3 (aq) + NaCl(aq) →
AgCl(ppt) + NaNO3(aq)
Question 6
Give an
example of each of the following chemical changes.
(a) A reaction involving
(i)
Change of state
(ii)
Formation of precipitate
(b)
An
exothermic and an endothermic reaction involving carbon as
one of the reactants.
(c)
A
reaction where colour change is noticed.
Answer
6
(a) (i) Change of state
Ammonia gas reacts with HCl gas to give solid ammonium
chloride.
NH3
(g) + HCl(g)
 NH4Cl(s) NH4Cl(s)
(ii)
Formation of precipitate
When a Aq, sol.of silver nitrate is
added to a Aq, sol.of sodium chloride, a white insoluble
substance, silver chloride, is formed.
AgNO3
(aq) + NaCl
(aq) → AgCl
(ppt) + NaNO3
(aq) (b) Exothermic
reaction:
When carbon burns in oxygen to form carbon dioxide, a lot of
heat is produced. C +
O2 → CO2 + Heat Endothermic reaction:
When carbon is heated with sulphur at high temperature,
liquid carbon disulphide is formed.
C + 2S CS2
(c)
Colour change
A few pieces of iron are added into
a blue coloured copper sulphate solution; the blue colour of
copper sulphate fades and eventually turns into light green
due to the formation of ferrous sulphate.
Fe + CuSO4 → FeSO4
+ Cu
Question 7
Define exothermic and endothermic
changes. Give two examples in each case.
Answer 7
Exothermic reaction:
A
chemical reaction in which heat is given out is called an
exothermic reaction. Example:
When carbon burns in oxygen to form
carbon dioxide, a lot of heat is produced.
C + O2 → CO2 +
Heat
When hydrogen is burnt in oxygen,
water is formed and heat is released.
2H2 + O2---à
2H2O + Heat
Endothermic reaction:
A
reaction in which heat is absorbed is called endothermic
reaction.
Example:
When carbon is heated with sulphur
at high temperature, liquid carbon disulphide is formed.
C + 2S---à
CS2
When nitrogen and oxygen are heated
together to a temperature of about 3000°C, nitric oxide gas
is formed.
N2
+ O2 ---à+
2NO
Question 8
State the effects of endothermic and
exothermic reactions on the surroundings.
Answer
8
Exothermic reactions are spontaneous and warm their
surroundings with the release of heat energy.
Endothermic reactions absorb heat
energy from their surroundings and cause their surroundings
to cool down.
Question 9
Define:
(a)
Photochemical reaction
(b)
Electrochemical reaction
Give one example in each case.
Answer
9
(a)
It
is a reaction which occurs with absorption of light energy.
Example: Photosynthesis
(b)
It
is a reaction which occurs with absorption of electrical
energy.
Example: Acidulated water breaks
into hydrogen and oxygen
Answer 10
(a)
NaCl(aq)
+ AgNO3(aq)
→ AgCl(aq)
+ NaNO3(aq)
(b)
Pb(NO3)2 + 2KI →2KNO3 + PbI2
(c)
CuCO3
CuO(s)
+ CO2
(g)
(d)
2Pb (NO3)2 2PbO + 4NO2 + O2
(e)
4NH3 + 5O2 4NO +6H2O
Question 11
What do
you observe? When (a)Lead nitrate is heated.
(b)Silver chloride is exposed to
sunlight.
(c)Hydrogen peroxide is exposed to
sunlight.
(d)H2S gas is passed
through copper sulphate solution. (e)Barium chloride is
added to sodium sulphate solution (f)Water is added to the
quick lime.
(g)Sodium chloride Answer is added
to silver nitrate solution.
Answer
11
(a)Lead
nitrate decomposes on heating, leaving a yellow residue of
lead monoxide, and brown nitrogen dioxide and colourless
oxygen gases are evolved.
(b)Due to thermal decomposition,
silver chloride breaks down into silver and chloride.
(c)Hydrogen peroxide breaks down to
form water and oxygen gas along with heat energy.
(d)When hydrogen sulphide is passed
through a blue Answerof copper sulphate, a black precipitate
of copper sulphide is obtained, and sulphuric acid so formed
remains in the solution.
(e)A white insoluble precipitate of
barium sulphate is formed.
(f)Quick lime reacts vigorously with
water to produce slaked lime, i.e. calcium hydroxide.
(g)When sodium chloride is added to the silver nitrate
solution, a white curdy precipitate of silver chloride is
formed.
Question 12
(a)
a
carbonate which do not decompose on heating.
(b)
a
nitrate which produces oxygen as the only gas. (c) a
compound which produces carbon dioxide on heating (d) a
nitrate which produces brown gas on heating.
Answer
12
(a)
Sodium carbonate
(b)
Sodium nitrate
(c)
Zinc carbonate
(d)
Lead nitrate
Class 9 subject Biology chapter3 "Tissues"
Ans1.Meristematic tissue and Permanent tissue.
Ans2.(i) permanent tissue.
(ii) parenchyma.
(iii) Collenchyma.
(iv) Xylem and phloem.
Ans3.(i)True.
(ii)True.
(iii)True.
(iv) false, The Sclerenchyma consists of dead cells.
Progress Check(pg-30)
Ans1(i) Epithelial tissue
(ii) Glandular epithelium
(iii)Muscular tissue
(iv) Nervous tissue
Ans2(i)Ciliated columnar epithelium is located in the
lining of the windpipe(trachea).
(ii)Elastic cartilage is located at the tip of the nose and
external ear.
(iii) Unstrained muscles are located in the walls of the
intestine and muscles of the iris of the eye.
Ans3(i)cartilage: It is elastic, on-porous and has no
blood vessels and nerves.
(ii)bone: It is hard, porous and has rich supply of blood
vessels and nerves
(iii) striated muscles: They are under the control of our
will. They are made of long fibres which are nucleated and
striated.
(iv)cardiac muscles: They are involuntary in function and
found only in the walls of the heart.
Ans4(i)true
(ii)false, axons bundled together form a nerve
(iii)false, cardiac muscles do not
get tired soon.
(iv)false, epithelial cells leave no space in between
(v)false, perikaryon is the cell body of a nerve cell
(vi)false, muscles of the iris of the eye are involuntary
type.
(vii)true
Review questions
Ex. A
1(c)parenchyma
2(a)fibrous connective tissue
3(a)meristem-actively dividing cells
4(d) chlorenchyma
5(d)layers of xylem in a stem
6(d) sclerenchyma
7(c)tendon
8(d) involuntary and striated
Ex. B Very short answer type:
And1(a)Apical meristem
(b)protective tissue
(c) glandular epithelium
(d)connective tissue (ligament)
(e) conducting tissue
(f) sclerenchyma
Ans2.Parenchyma is the least specialized tissue
located in plants.
Ans3.(a) tissue
(b)parenchyma
(c)lateral meristem or cambium
Ans4(a)At the tips of roots, stem and branches
(b)At the tip of nose and ear
(c)in the mouth and nasal cavity
(d)in stems and veins of leaves
(e)in the lining of windpipe (trachea)
(f)at the end of two bones
And5(a) epithelial tissue
( b)cuboidal epithelium
(c)nerve cell(neuron)
(d)ciliated epithelium
Ex. C Short answer type
Ans1Ciliated epithelium is found in the inner lining
of windpipe(trachea).
The cilia present in it keep lashing and move the materials
out which enter these regions.
Ans2 Nervous tissue: It is made up of elongated cells
called neurons. This tissue is concerned with the perception
and responses of animals.
Nervous system: It consists of brain, spinal cord and the
nervous tissue. This system controls and coordinates all the
systems and parts of your body.
Ans3Tissues found in human heart are:
Epithelial tissue, connective tissue, muscular tissue and
nervous tissue
Ans4.No, we cannot consider because tissue is a group of
cells performing a particular function but cluster of eggs
cannot perform a specific function rather they perform it
individually
Ans5.The three types of muscles found in the human
body are:
(1)Striated muscles: found in arms and legs.
(2)Unstrained muscles: found in intestine and stomach.
(3)Cardiac muscles: found in heart.
Ex D Long answer type
An1(a)Cell and tissue:
Cell: It is the structural and functional unit of life.
E.g. nerve cell
Tissue: It is the group of similar cells performing a
specific function.
E.g. connective tissue
(b)organ and organism:
Organ: several tissue make up an organ. They perform
function within your body.
E.g. heart, liver
Organism: several organ system make up an organism.
E.g. humans, animals
(c)Organ and organelle
Organ: several tissue make up an organ. They perform
function within your body.
E.g. heart, liver
Organelle: little organs found within the cell. They perform
function within the cell
E.g. ribosomes, mitochondria.
(d)Organ and Organ system.:
Organ: several tissue make up an organ. They perform
function within your body.
E.g. heart, liver
Organ system: several organs together performing a specific
life process form an organ system
E.g. circulatory system
And(a)Parenchyma and collenchyma:
Parenchyma: They are the thin walled cells which may be
oval, or round in shape. These cells help in the storage of
food.
Collenchyma: They are the elongated cells with thickened
cell wall at the corners. These cells give mechanical
support to the parts of the plant
(b)Meristematic tissue and permanent tissue:
Meristematic tissue: These are made up of actively dividing
cells. Cells are small usually cuboidal.
Permanent tissue: These are made up of cells which have lost
their ability to divide.
(c) Sclerenchyma and parenchyma:
Sclerenchyma: They are made up dead cells. Here, cell wall
is thick. They give strength to the parts of the plants.
Parenchymal: They are made up of living cells. Here, cell
wall is thin. These cells help in the storage of food.
(d)cells of involuntary muscles are spindle shaped
with tapering ends. Dark and light bands are not present.
These muscles don't work according to our will while cells
of voluntary muscles are long. Striations that is dark and
light bands are present in these cells. These muscles work
according to our will.
(e)fibres of voluntary muscles are long cells
constituting voluntary muscles. They are not branched. They
are found in arms, legs, face, neck, etc.
Cardiac muscles are made up of short cells but they are
branched They are found in the heart.
Ans. E Structured/application questions
1 (a)The given diagram is the phloem tissue of
plant as it contains the cellular components of phloem.
(b)1.sieve cell or sieve tube
2.phloem parenchyma cells
3.companion cells
4.sieve plate
(c)This tissue is likely to be found in the stem and
leaves of plants.
(d)sieve tubes helps in the transport of food from
leaves to storage organs and other parts of the plant.
Phloem parenchymal helps in the storage of starch and fats.
Companion cells help in the functioning of the sieve tubes.
Sieve plate allows the transportation of food as it contains
perforations.
Ans2(a)This cell is nerve cell or neuron.
(b)1-Cellbody
2-Axon
3-Nucleus
4-Nissl granule
5-Neurolemma
6-Axon endings
(c) This cell is likely to be found in nervous system. Nerve
cells conduct impulses from one part of the body to other.
Class
9 physics chapter2
Motion in one dimension.
Exercise 2 (a)
Q1.
Differentiate between scalar and vector quantities, giving
two examples of each.
Ans Scalar
quantities: These are physical quantities which are
expressed only by their magnitudes.
Examples:length and mass.
Vector quantities:These physical quantities requires the
magnitude as well as direction to express them.
Examples:Force and weight.
Q2. when is
a body said to be at rest?
Ans A body
is said to be at rest if it does not change its position
with respect to it's surroundings.
Q3
When is a body said to be in motion?
Ans A body
is said to be in motion if it change it's position with
respect to it's surroundings.
Q4
What do you mean by motion in one direction?
Ans When a
body moves along a straight line path, it's motion is said
to be one dimensional motion.
Q5
Define displacement. State it's unit.
Ans The
shortest distance from the initial to the final position of
the body. It's S. I. Unit is metre(m).
Q6
Differentiate between distance and displacement.
Ans from
book, page no. 29.
Q7
Can displacement be zero even if distance is not
zero? Give one example to explain your answer.
Ans yes
,displacement can be zero even if distance is not zero. For
example: if a body moves in a circle,
then displacement in one rotation is zero but
distance covered in one rotation is circumfrence of the
circular path.
Q8 Define
velocity. State it's s. I. Unit.
AnsThe
velocity of a body is the distance travelled per second by
the body in a specified direction. Its S.
I unit is ms-1.
Q9
Define speed. What is its S. I. Unit?
Ans The
speed of a body is the rate of change of distance with time.
Its S. I. Unit is ms-1.
Q10
Distinguish between speed and velocity.
Ans from
book, page no. 32.
Q11 When is
the instantaneous speed same as the average speed?
Ans When the
body moves with uniform speed.
Q12
Distinguish between uniform velocity and variable velocity.
Ans uniform
velocity:1when a body covers equal distance in a straight
line, in equal interval of time.
2.
in this case, direction of motion remains same.
3. eg.a body
moving with a constant speed in a straight line has uniform
velocity.
Variable
velocity:1.when a body covers unequal distance in equal
intervals of time in a straight line.
2. in this
case, direction of motion changes.
3.
eg.circular motion is example of variable velocity.
Q13
Distinguish between average speed and average velocity.
Ans Average
speed:
1. it is the
ratio of total distance travelled by the body to total time
taken to travel that distance.
Thus,
Average
speed=total distance travelled/total time taken.
2. It is a
scalar quantity.
Average
velocity:
1. if the
velocity of a body moving in a particular direction changes
with time, the ratio of displacement
to time taken in the entire journey is called its
average velocity. Thus,
Average
velocity=Displacement/total time taken
2. it is a
vector quantity.
Q14 Give an
example of motion of a body moving with a constant speed,
but with variable velocity. Draw a
diagram to represent such a motion.
Ans The
motion of a body
in a circular path even with constant speed, but with
variable velocity because in
a circular path, the direction of motion of body
continuously changes with time. Its velocity changes at a
uniform rate. At any instant, it's velocity is along the
tangent to the circular path at that point. Fig. 2.5 from
book
Q15Give an
example of motion in which average speed is not zero, but
average velocity is zero.
Ans if a
body starts its motion from a point and comes back to the
same point after a certain time, the displacement is zero,
so the average velocity is also zero, but total distance
travelled is not zero so the average speed is not zero.
Q16Define
acceleration. State it's S. I. Unit.
Ans
Acceleration is the rate of change of velocity with time.
Its S. I. Unit is ms-2.
Q17Distinguish between acceleration and retardation.
Acceleration
(1). Rate of change of velocity with time is called
acceleration.
(2). A body
falling towards the earth has positive acceleration.
Retardation
(1). When velocity of body decreases with time, the motion
is said to be decelerated or retarded.
(2). A ball
thrown vertically upward has retardation.
Q18Differentiate between uniform acceleration and variable
acceleration.
Ans Uniform
acceleration:(1) The acceleration is said to be uniform when
equal change in velocity take place in equal interval of
time.
(2) The
motion of body under gravity is an example of uniform
acceleration.
Variable
acceleration:(1) if change in velocity is not same
in the same interval of time, the acceleration is
said to variable.
(2) The
motion of vehicle on a crowded road is with variable
acceleration.
Q19What is
meant by the term retardation? Name its
S. I unit.
Ans Negative
acceleration is called retardation. Its S. I. Unit is ms-2.
Q20 Give
example of each type of the following motion:(a) uniform
velocity:The rain drops reach on earth's surface falling
with uniform velocity.
(b) variable
velocity:the motion of a body in a circular path.
(C) variable
acceleration:the motion of a vehicle on a crowded or hilly
road.
(d) uniform
retardation:if brakes are applied on a train approaching a
station to stop it.
Q21 Define
the term acceleration due to gravity. State its average
value.
Ans When a
body falls freely under gravity, the acceleration produced
in the body due to earth's
gravitational attraction is called acceleration due
to gravity(g). The average value of g is 9.8ms-2.
Exercise
2 (b)
Q1
What
informations about the motion of a body are obtained from
the displacement time graph?
Ans (1)The
slope of displacement-time graph gives the velocity.
(2) The
displacement-time graph is a straight line inclined to the
time axis. It shows linear relationship
between the displacement and time.
(3) The
displacement time graph is a straight line parallel to time
axis mean the body is at rest.
Q2. (a)
What does the slope of a displacement time graph
represent?
Ans The
slope of a displacement time graph gives velocity.
(b) Can
displacement time sketch be parallel to the displacement
axis? Give reason to your answer.
Ans No, the
displacement time graph can never be a straight line
parallel to the displacement axis because
such a line would mean that the distance covered by
the body in a certain direction increases without
any increase in time(I.e.velocity of the body is
infinite) which is impossible.
Q3
Draw a displacement time graph for a boy going to
school with a uniform velocity.
Ans from the
book at page no 37 figure 2.8
Q4
State how the velocity time graph used to find (1)
the acceleration of a body
Ans:
acceleration=velocity÷time, if slope is positive mean
accelerated motion. If slope is negative mean
retardation. If slope is zero means acceleration is
zero.
(2) the
distance travelled by the body in a given time(3) the
displacement of a body in a given time.
Ans The
distance travelled by the body in a given time can be
obtained by finding area enclosed between the
velocity time graph and X axis gives displacement of
the body. Total distance travelled by body is their
arithmetic sum(without sign)
The
total displacement is obtained by adding them numerically
with proper sign.
Q5
Draw a velocity time graph for a body moving with
initial velocity u and uniform acceleration a. Use
this graph to find distance travelled by the body in
time t.
Ans from
book at page no. 51,figure 2.45.
Distance travelled by body in time t=area of Trapezium OABD
=area of rectangle OAED+area of triangle ABE
Or
S=OA*OD+1/2*BE*AE=ut+1/2(v-u) *t
=ut+1/2at2.
Q6 Draw the
shape of the velocity time graph for a body moving(a)
uniform velocity, (b) uniform
acceleration.
Ans from
book at page no. 40,fig 2.13 for part a
and fig 2.14 for part b.
Q7 Draw a
graph for acceleration against time for a uniformly
accelerated motion. How can it be used to
find the change in speed in certain interval of time?
Ans fig.
2.19 at page no. 42
Since acceleration=velocity÷time
therefore, acceleration×time=change in speed. So in the
given graph the area enclosed between the
acceleration time sketch and time axis gives change
in speed of the body.
Q8
Draw a velocity time graph for the free fall of a
body under gravity, starting from rest. Take g=10ms-2.
Ans figure
2.22 at page no. 43
Q9
A body falls freely from a certain height. Show
graphically the relationship between the distance
fallen and square of time. How will you determine g
from this graph?
Ans
fig. 2.24 at page no. 44
The value of g can be obtained by doubling the slope
of the S-t2
graph for a freely fallig body.
Please learn and write on your copies.
History
Class 9 - Chapter 2
structured questions
( based on 2019
edition)
1. With reference to the sources of information about the
Vedic age......
a. The Vedas. b. The Epic c. Role of iron
implements
Ans- a. The social, economic, political and religious
aspects of the life of the people came to be reflected in
the Vedic literature. Thus, Vedas became the storehouse of
knowledge. The Vedic literature was written in Sanskrit. The
term, Veda' has been derived from the Sanskrit word, 'vid'
which means knowledge.
.
b. The Epics serve as the main source of information on the
political institutions and the social and cultural
organisation of the Epic Age
(ii) They provide information on various Aryan Kingdoms,
their armies and the weapons they used.
iii.They reveal the high ideals of family life of the
Aryans.
c. i. Agriculture: Discovery of iron gave the Aryans
new implements like axes to clear the forest and cultivate
the land.
(ii) Occupation: The use of iron gave rise to new trades by
providing durable implements like saws, chisels, hammers,
nails and tongs.
(iii) Defence: Because of its durability and easy
availability, iron was extensively used in making weapons
like swords, armours and shields.
2. With reference to Vedic Literature, answer the following
questions:
(a) List the categories into which Vedic literature is
divided.
(b) Give a brief account of the four Vedas.
(c) What are known as Brahmanas and Aranyakas?
Ans- a. The Vedic literature can be classified into the
following categories: (i) The four Vedas, i.e., the Rig,
Sama, Yajur and Atharva Vedas and their Samhitas. (ii) The
Brahmanas attached to each Samhita (iii) The Aranyakas (iv)
The Upanishads.
b. i. The Rig Veda : It is the oldest religious text of the
world. It is said to have been composed during the early
Vedic Period . The hymns are dedicated by the sages to Gods
ii.
) The Sama Veda: In this veda some of the hymns are borrowed
from the Rig Veda, These hymns were meant to be sung at the
time of the sacrifice by the priests]
(iii) The Yajur Veda: (It deals with hymns recited during
the performance of Yajnas
(iv) The Atharva Veda: The hymns contained in this Veda deal
with magic and charm. Most of the hymns are taken from the
Rig Veda.
c. The Brahmanas
Written after the Vedas as their simple commentary, the
Brahmanas are in prose. They explain the social and
religious importance of rituals as well as the value of
sacrifices.
The Aranyakas
They are known as forest books' written for the guidance of
the hermits and the students living in forests.
3. With reference to the Society during the Vedic Age,
answer the following
(a) Explain briefly the class divisions that existed in the
society. (b) Explain the four stages in the life of an
Aryan.
(c) State the position of women in the Vedic period.
a. People followed different professions which became
hereditary in course of time. This resulted in the division
of society into occupational stages classes. Gradually, this
took the form of caste and the present caste system emerged.
In the Later Vedic Period, Brahmins, Kshatriyas, Vaishyas
and Shudras became four distinct castes or Varnas.
b. i. The The Brahmacharya Ashrama lasted upto the age
of 25 years. During this period, the pupil was expected to
acquire knowledge in the gurukul and observe strict
discipline.
ii.Grihastha Ashrama,25 to 50 years
Man was supposed to marry and raise a family. As a
householder, he was to take responsibility of maintaining
his family.
iii. The third stage was Vanaprastha Ashrama,50 to 75 years.
During this period man was expected to retire from worldly
life and acquire spiritual and philosophical knowledge.
iv. The Sanyasa Ashrama,75 to 100 years.
This was the period of renunciation. Man had to leave
everything forever and go into meditation in order to attain
moksha or salvation.
c. POSITION OF WOMEN
During the Rig Vedic Period women were respected. The
institution of marriage had become sacred. The daughters
were given freedom to choose their husbands.
In the Later Vedic Period, there was significant decline in
the status of women.They did not enjoy the right to
property. The freedom to choose husbands by women was
curtailed.
4. With reference to economic organisation of the people in
the Vedic Period, answer te following questions:
(a) State the methods used by the Aryans in agriculture.
(b) Why was domestication of animals important to the
Aryans? (c) How was trade managed during this period?
Ans- a. Agriculture: Discovery of iron gave the Aryans new
implements like axes to clear the forest and cultivate the
land. Thus, agriculture became their important occupation.
With the use of iron plough-heads, sickles and hoes, they
could bring vast tracts of land under cultivation. The
production of more rice, wheat, barley, vegetables and
fruits improved their standard of living.
b. In the beginning the main occupation was domestication
agriculture was secondary. Cattle was the important source
of wealth.Cows were domesticated and milk and milk products
like curd, butter, ghee, etc. were used.
c.Trade:During the Later Vedic phase agricultural surplus
led to trade, giving rise to markets from which developed
towns and cities. Thus, trade became the pivot around which
the whole town and city life moved. Although the Aryans had
introduced coins, barter system was still dominant in trade
with other countries.
5. With reference to the picture given, answer the following
questions:
(a) Identify this ancient education system. Persons
belonging to which ashrama of life attended this?
(b) Describe briefly the life in this ancient education
institution.
(c) What do you think are the advantages and disadvantages
of studying in this educational setup?
Ans- a. This system was known as Gurukul system of
education.
The person belonged to the Brahamacharya Ashrama.
b. The student was required to do household chores for his
teacher. He had to get up early in the morning, take a bath
and chant the Vedic mantras. Most of the teaching was done
orally. At the completion of the education, a student used
to give guru dakshina – a gift to his teacher.The main
object of education was to bring about physical, mental and
spiritual development of the pupils.
c. Advantages;
i. It was focused on all round development of a student like
mental, physical etc.
ii. Pupils learnt to live a disciplined life.
Disadvantages;
i. Pupils had to left the home at tender age which deprived
them from parental love.
ii. There was no formal system of evaluation.
History
Chapter 1 structured questions
Q1
Answer
a.
The great bath: it indicates the following
1. the art of building had reached a high degree of
perfection.
2. There might have existed a ruling class that could
mobilize labour.
3. The importance attached to ceremonial bathing
4. The efficient planning in the structural features
relating to water supply
b.
The seals:
1.
These are used by the Harappans to show their artistic
skills.
2.
These provide useful information about script, trade,
religion and beliefs.
3.
These were used by the traders to stamp their goods.
C.
script : the harappans used a script which is regarded as
pictographic since its signs represent birds, fish and
varieties of the human form. The number of signs of harappan
is known to be between 375 and 400.
Q2. answer:
a.
In
1921, dayaram sahni an officer in the archaeological
department. Survey of India got ruins dug out, around
harappa. In 1922, R.D Banerjee along with a biddhist monk
found the city of mohenjo daro under a mound. Later, sir
john marshall ordered large scale excavations.
b.
The entire area of the harappan civilization is triangular
in shape covering an area of about 1,299,600 sq. km,
extending from
sutkagendor in balochistan in the west to alamgirpur, in
ganga yamuna doab and from manda in jammu in north to
bhagatrav in narmada estuary in the north.
c.
Manda, banawali, kalibangan, alamgirpur, lothal rupar and
rangpur.
Q3. answer:
a.
Town planning: it is the most remarkable feature of the
Harappan civilisation. The indus cities were set up on a
grid-pattern, consisting of regular planning with division,
alignment of streets, planning of the houses and public
building with the provision of thoroughfares.
b.
Special features of
the houses: the houses at street corners were rounded to
allow carts to pass easily and the house drains emptied all
waste water into the street drains.
c.
Common elements:
given on page no. 9 shaded portions.
Q4. answer:
a.
A
large number of stone images have been found; out of these
the statue of a yogi draped with a shawl is well-known.
Bronze statues of
a dancing girl, animals and carts etc. are
noteworthy.
b.
Harappans produced their own characteristics pottery which
was made glossy and shining. Earthen vessels and pottery
crafted on the potter’s wheel, were decorated with black
geometrical designs.
The large jars with narrow necks and red pots with
black decoration bear evidence of their artistic skill.
c.
The sculpture in metal was done through the special lost wax
process. In this process wax figures were covered with a
coating of clay. Then the wax was melted by heating and the
hollow mould thus created was filled with molten metal which
took the original shape of the object.
Q5
answer:
a.
The seal given in the picture is a pashupati seal.
The seals were used for trade in the vast area of indus
valley, as these have been found from various spots spread
over the civilisation sites. The seals with short
inscriptions gave some message which cannot be deciphered
yet.
b.
The seals were made of terra-cotta, steatite, agate etc.
c.
The seals reveal the mythical and religious beliefs. The
figures carved in the seals depict the worship of mother
goddess and Pashupati and various animals, trees etc.
Q6
answer:
a.
The picture is of a great bath.
1.
It
has a large rectangular tank in a courtyard surrounded by a
corridor on all four sides.
2.
There are two flights of steps one in the north and the
other in the south leading into the tank.
b.
. same as first a.
C.
the citadel: the raised area of each city was called the
citadel. It owed its height to the buildings constructed on
mud brick platforms. The citadel had the houses of the
ruling class and important buildings like the great bath,
the granary, the assembly hall and the workshops.
Chapter 3 jainism and
buddhism
Q1
answer
a.
Same as 1 short answer.
b.
Same as 2 short answer.
c.
Jatakas : the jatakas tales written in pali language refer
to the previous birth of lord buddha. They also throw light
on the political, economic and social conditions ranging
from the fifth to second century.
Q2. answer:
a.
Monopoly of the priests, expensive rituals, difficult
sanskrit language and rigid caste system universally
affected the life and feelings of the common people. Some
kings had also realised certain social evils and
so patronised Jainism and Buddhism for a better
pattern of life and simple rules for spiritual uplift.
Ahimsa and the safety of animals were also prefered by
farmers.
b.
Causes for the rise of jainism and buddhism :
1.
Corruption in the religion: in order to extract money,
priests encouraged ordinary people to perform yajnas and
conduct household rites beyond their means.
2.
Rigid caste system: the division of society becomes rigid.
It did not allow any mobility. A person of one caste could
not become a member of another society.there were
restrictions on the basis of caste.
3.
Difficult language: vedic literature was written in sanskrit
which was very difficult to understand and it gave birth to
the monopoly of brahmins over religion. They started
exploiting other castes.
4.
Political impact: the republics of shakays, vajjis, and
mallas embraced buddhism ashoka and kanishka made buddhism
their state religion.
c
. when bhadrabahu took jainism to karnataka; at the
same time sthulibhadra in magadh spread jainism with
different ideas. So after the first jain council held around
300BC, jainism was divided into two groups or sects.
Q3. answer:
a.
Mahavira was the twenty fourth and the last of the
tirthankaras. He made Jainism popular and systematic.
Mahavira was born in kundagram near vaishali in bihar in the
2nd half of the 6th century B.C.
b.
He
got absorbed in spiritual pursuits from early childhood. At
the age of thirty, after the death of his parents,Mahavira
renounced the world and became ascetic. He practised severe
penance for 12 years and attained supreme knowledge.
c.
Mahavira conquered all worldly desires and was named as
jina, which means the conqueror.
Q4. answer:
a.
Nine truths:
1.
Punya (results of good deeds).
2.
Pap (sin)
3.
Ashrav (good deeds)
4.
Sanvar (hindrance in the way of karma)
5.
Nirjara (destruction of the karmas)
6.
Moksha (salvation)
7.
Jiva (living things)
8.
Ajiva (non living things)
9.
Bandha (bondage).
b.
. doctrines:
1)
triratnas : right faith, right knowledge, right conduct.
2)
Karma: good deeds provide moksha.
3)
Equality: universal brotherhood.
4)
Eternal soul: soul is immortal.
5)
Belief in penance: sacrifice of physical desires,
6)
Salvation: freedom from life and death.
C.
causes
1)
Jainism preached rigid austerity.
2)
The religion did not spread to the foriegn countries.
3)
Jainism did not get royal patronage.
4)
Hinduism revived during the Gupta period.
Q5
a.
Gautam buddha was the son of sakya king, suddhodana of
kapilvastu. He was born in 563 B.C at lumbini. After
studying under renowned teachers of rajgriha, he went to
gaya and practised severe penance and at the age of thirty
-five he attained enlightenment.
b.
After enlightenment he preached his first sermon at deer
park sarnath this incident is known as
dharmachakrapravartana.
c.
He
established a bodh sangha at magadha. He received the
patronage of several rulers of magadha, kosala and kosambi
and followed by a large number of followers from all classes
of society.
Q6
Answer:
a.
Membership: the members of monastic order required to
renounce the world before joining the sangha. The members
had to take the oath, they also had to undergo training for
10 years.
b.
1. To speak the
truth.
2. Not to harm the creatures.
3. Not to keep money.
c.
The principles of Buddhism have a deep effect on social
life. Which gave impetus to weaker sections of the society
to fight for their rights and survival. The social-religious
reform movements were the plus points and the solid
political ground.
Q7
answer:
a.
1)
Gautam buddha founded Buddhism. 2) Mahavira founded the
jainism.
b.
Main teachings
1.
Ahimsa i.e non violence.
2.
Asateya i.e not stealing.
3.
Satya i.e not telling a lie.
4.
Apargriha i.e not possessing property.
5.
Brahamcharya i.e practising chastity.
Four noble truths:
1.
The world is full of suffering.
2.
The suffering has a cause.
3.
Desire is the cause of suffering
4.
If
desire is stopped, suffering can also be stopped.
C.
similarities:
1.
Both did not accept the vedas.
2.
Non-violence was their creed.
Dissimilarities:
1.
Buddhism followed a middle path but Jainism believed in hard
penance.
2.
It
is silent about the existence of god whereas jainism denies
the existence of god.
GEOGRAPHY
STD
IX
CHAPTER 5
LANDFORM OF THE EARTH
Ans 1 Plains are comparatively flat and a level surface of
land with least
in its highest and
lowest points
Ans 2The process of
mountain building that occurs on large scale. Literally, the
birth of mountain.
Ans 3 The different types of mountains are fold mountain,
block mountain and volcanic mountain.
Fold mountain- These mountains have been formed due to the
sedimentary deposits and large scale earth movements caused
by tectonic force . e.g. Himalayas,
Alps, Andes etc.
Block mountain – These mountain have been formed due to
faulting in the ground surface. E.g. Vosges and black forest
Mt.
Volcanic mountain- These mountain have been formed by the
accumulation of lava around the crater which is thrown out
during a volcanic eruption.e.g.fujiyama and Mauna loo.
Ans 4 An extensive almost flat topped region is called
plateau.
Ans 5 The various typed of plateaus are:
[1] Intermundane plateau eg Columbia plateau in north
America.
[2] Piedmont plateau eg Patagonian plateau in south America.
[3] Volcanic plateau eg Deccan plateau in India.
Ans 6 Advantages of plains are :
1-
They are extensive area of lowland.
2-
Many of them are smooth or have a gentle undulating surface.
Ans 7 Following are the types of depositional plains.
1 Alluvial plains These are formed by the depositional work
of rivers and are divided into three categories.
a-piedmont alluvial plains-These are formed at the foothill
of the mountains, due to the deposition of the material like
cobbles, pebbles, boulders
B-Flood plains- A low lying area near a stream.
C – Delta plains-These are formed at the mouth of a river in
the lower valley
2-Loess plains- These are formed by the depositional work of
wind.
3-Glacial depositional plains-these plains are formed by the
depositional work of glaciers
Ans 8 The main uses of mountains are –
1-They are the storehouse of minerals like coal, iron ore,
gold etc.
2-They are the source of rivers.
3-They are important for their natural vegetation.
4 They are a natural barrier and also form an important
political boundary
5 They have a great impact on the climate.
Ans 9-The difference between intermundane plateau and
volcanic plateau;
INTERMONTAIN PLATEAU are surround by mountain. Their surface
show an variety of topographic features for eg. Plateau of
Tibet and Mexican plateau.
VOLCANIC PLATEAUS are formed by lava flow from volcanic
eruption for eg. The plateau of peninsular India.
Ans 10 Plains are flat and level surface of land with least
difference in its highest and lowest point .They are
extensive area of low land.
AND Plateau is an extensive flat topped region.
Ans 11 A mass of very high rock rising to great height above
the surrounding areas is called mountains.
Ans 12 CHARACTERISTICS OF YOUNG FOLG MOUNTAIN:
[1] These have been formed in the most recent phase OF
Mountain building.
[2] They have rugged relief feature.
Ans 13 The mountains of young fold type are characterized by
ruggedness of relief and rounded contours of mountain areas
which have been subjected to weathering agents for long
period of time.eg. the alps and the Scottish highlands.
Ans 14 Alluvial plains are formed by the depositional work
of river.
Ans 15: [1] Fold mountains eg Himalayas.
[2] Block mountains eg African rift valley.
[3] Volcanic plateau eg Deccan plateau.
[4] Structural plain eg. The great plain of USA.
[B] DEFINE THE FOLLOWING :
[1] same as ans 9
[2] Block mountain- see ans 3.
[C] DIAGRAMS:
[1] FIG 5.5
[2]FIG 5.11
Class-9
Geography Ch-2 Latitudes and Longitudes Ex-A
Answers the following:-
1]We need to locate places on earth because earth is a huge
planet & to know it properly
we need to locate place.
2]The latitude of a place is the
angular distance of that place north or south of the
equator,as measured from the
centre of the earth.
3]Pg.-30 from technical terms.
4]Because there is no land mass
beyond 90° north & 90° south.
5]These lines are called
meridians,meaning "mid-day" as all the places on the same
meridians have their noon at the
same time.
6]pg-29,content analysis 4th
point
7]phg-29-30,content analysis 6th
point
8]The distance between each
latitude,is approximately 111KM,as earth represent 360°
and the circumference of te earth
is approximately 40000KM. THUS,40000/360°Pg-30
content b=111KM
9]The north temperate lies
between 23 1/2° N to 66 1/2° N and south temperate
zone 23 1/2° S to 66 1/2°.stretch
from torrid zone to frigid zone
10]Because torrid zone receives
direct rays of the sun.
11]Because of inclination of
earth's axis and recieve very slant rays of the sun.
12]Local time of a place is that
time when sun is overhead to meridian.It is fixed by
the apparent movement of the sun.
13] YES
14] 1°=4min
30°=4x30=120min or 2hrs
12noon +2hrs=14hrs or 2pm
15]If each city werre to keep the
time of its own meridian,there would be much diffrence
in local time betweeen one city
and another.Therefore, a system ofr standard time is
observed by all countries
16]Pg-30 from technical terms.We
have 24 time zones.
17]Because singapore lies in the
torrid zone and london lies in the temperate zone.
18]pg-30 content analysis g
point.international date line is a line concerned with
the dates of the calender and
adopted international.
19]Due to its large east west
extent.
20]Except the equator,other
parallels of latitudes are known small circle.
21]Because these latitudes do not
divide the earth into two equal half.
22]If each town,village anf city
in the world had kept its on time it would have cause
utter confusion,as the clocks and
watches would have to be constantly altered as one
travelled from one place to
another.
23]Pg-30 from technical terms.
24]Earthosthens.
25]Important climatic zones of
the world are- The Torrid or Tropical zone,Temperate zone
and Frigid zone.
26]The earth takes 24 hrs to run
throw 360 degrees.The sun appears to moves at the rate
of 15° in 1hr or 360°in 24hrs or
1°in 4 minutes.
27]The intrection of latitude and
longitude at right angle is the extact position of a
place on the earth's
surface.latitude give us the location of any place in
northen
and southern hemisphere while
longitude help us locate the place in eastern or
western hemisphere.with the help
of these intersecting line we can locate the
position of any place on the
globe or map.
28]
1-Latitude
2-Longitude
3-40°North
29]
A-Local Time-Local time of a
place is that time when sun is overhead to the meridian.
it is fixed by the appearent
movement of the sun.
Standard Time-Most country adopt
their standard from the local time of their central
meridian.for eg-India follows the
local time of its central meridian 82 1/2° East
which passes throw Allahabad.
B-Parallel run from east to west
and never intersect with each other whereas
meridians run from north to south
and intersect at north and south poles.
C-Equator separates the Northern
and Southern hemispheres.The equator is at 0°latitude.
the prime meridian separates the
Eastern nad Western hemispheres.The prime meridian
run through Greenwich,England and
is at 0°Longitude.
ex-B,Define the terms:-
1,2,3,4 from technical terms
pg-30
5,6 same ans 29a
7-In india the longitude 82 1/2°
East is selected as standard meridian,which passes
which passes through
Allahabad.since its local time is taken as standard Time,the
whole country follows it,it is
called indian standard time.
8-pg-30 content analysis F point
9-same ans 18
10-The 0°Latitude is known as
great circle.It divides the earth into two equal halves
11-same ans 20
12-Same ans 23
Ex-c
Distinguish between the
following:-
1-pg-29 table 2.1
2-from ex-b 10 and 11 define
3-from ex-b 8 & 7 define
EX-D
Give reasons:-
1-Because latituides are angular
distance of a place north and south to the equator and
longitude are the angular
distance of a place east and west to the prime meridian.
2-Due to large east-west extent.
3-To avoid cutting through the
continent.
4-Because the places east of
greenwich see the sun earlier and gain time or Because sun
rise in the east and set in the
west.
5-Because latitudes are parallel
to each other.
6-Because mumbai lies in the east
of prime meridian and london lies in the prime
meridian.
7-The difference between the
indian standard time and the greenwich is 82 1/2°,i.e.,
5 1/2 hrs.0° to 82 1/2°east =82
1/2°X 4=330 minutes or 5 1/2 hrs.
Ex-E
1-pg-21 fig.2.7
2-pg-23 fig.2.10
3-pg-20 fig.2.3
Dear students some questions are not given in new book. Here
we are adding few questions to cover inside portion.
Class 9th history and civics harappan
civilisation
I.
Short answer
questions:
QUESTION1. what is
meant by the term civilisation?
Answer:
Civilisation is
defined as an advanced stage of human cultural development
it ipmlies the use of superior technology and complex
economic relationships. Following are the traits which mark
a civilisation:
1.
Surplus food
2.
Division of labour
3.
System of writing
4.
Development of technology
Question2. mention
any three features that led to the emergence of civilisation
Answer:
1.
The ruins of the sites reveal that the
harappan people were primarily urban and their cities were
designed skillfully.
2.
The unique features of the city was its
elaborate drainage system. A brick-lined drainage channel
flowed alongside every street.
3.
The great bath was also unearthed. The
pool was filled with water taken from a well nearby. The
walls of the pool were made.
QUESTION3.
What are known as
bronze age civilisation?
Answer:
Man learnt the art
of mixing copper with tin of zinc to produce the alloy
called bronze. Bronze is hard and more ductile than copper
and is therefore, more suitable for the mannufacture of tool
and weapons. Because of the importance of bronze in the
growth of the civilisations, these civilisations are known
as the bronze age civilisation.
QUESTION4
Name the important
sources of information on the harappan civilisation
Answer:
The archeaological
remains
Seals, sculptures
QUESTION5.
Why did ancient man
start using bronze for making tools and weapons
Answer:
Ancient man start
using bronze for making tools ans weapons because bronze is
harder and more ductile than copper. Therefore, it is more
suitable for the manufacture of tools and weapons.
QUESTION6.
Why is harappan
civilisation called so?
Answer:
The harappan
civilisation is called so because the harappan site was the
first to be discovered in 1921 at the modern site of harappa
situated in the province of west punjab in pakistan.
QUESTION7.
What types of
weights and measures did the indus people use?
Answer:
The indus people
used sets of cubical stone weights. The basic unit was
16(equalto mmodern 14 grams). the larger weights were
multiples of 16 like 32,48,64,128 and so on. The smaller
ones were all fractions of 16.
QUESTION8.
Name one important
public building of indus valley civilisation and its
importance.
Answer:
The great bath is
one of the largest public buildings at mohen-jo-daro. It was
used by a large group of people while performing some
religious rituals.
QUESTION9.
Name the process by
which sculpture in metal was done
Answer:
It was known as lost
wax process.
QUESTION10.
QUESTIONwhat do you
know about the indus script?
Answer:
Indus scripts has
not been deciphered as yet. So the only source of script are
some seals and copper tablet. Seals display some sort of
pictorial writing. Besides this similar inscriptions have
been found engraved on copper tablets with figures of men
and animals.
QUESTION11.
Mention the types of
dress worn by the indus valley people.
Answer:
Most people used
cotton clothes. Rich people also used woollen clothes in
winter. The women’s dress included skirts, cloaks and scarfs
and the men’s dress was dhoti and shawl. Spinning wheels and
needles of that time prove the art of spinning was practiced
by the people.
QUESTION12
State two features
of the trade in the indus valley civilisation.
Answer:
1.
the harappan carried on considerable
trade in stone, metal, shell etc.
2.
They however, did not use metal money but
carried on all exchange through barter system
Question13.
Name three animals
depicted on pashupati seal
Answer:
Seals of pashupati
shows a three faced deity wearing a buffalo-horned head
dress, seated cross legged on a throne and surrounded by an
elephant, a tiger, a buffalo and a rhinoceros, with two deer
at his feet.
QUESTION14.
State any two causes
that led to the decline of the harappan civilisation
Answer:
1.
Floods and earthquake: it is held by some
scholars that floods in mohenjo-daro led to the abandonment.
2.
Attack: some historians believed that the
invading aryans destroyed the indus valley civilisation.
QUESTION15.
In what two respects
is harappan civilisation our greatest heritage?
Answer:
The harappan
civilisation present a basic ground of indispensible
heritage which imparts a soild imprint on the latter
civilisation e.g the way of making baked pottery, bricks,
beads jwellery etc the cultivation of cotton was adopted by
the egyptians after several centuries.
QUESTION16.
Give two
characteristic features of the citadel
Answer:
It owed its height
to the building constructed on mud brick platforms. The
citadel had the houses of ruling class and important
building like the great bath, the grannary, the assembly
hall and the workshops.
QUESTION17.
How were seals used?
What information do they give about harappan trade?
Answer:
The seals were used
by traders to stamp their goods. After a bag with goods was
tied, a layer of wer clay was applied on the knot, and the
seal was passed on it. These seals were found in different
regions. This indiacates that the harappan trade had spread
over a vast area.
QUESTION18
Briefly describe
granaries at harappa.
Answer:
In harappa there
were two rows of six granaries each. To the south of the
granaries at harappa working floors consisting of rows of
circula brick platforms were discovered. It is believed that
these floors were meant for threshing grain because wheat
and barley were found in the crevices of the floors.
QUESTION19.
Breifly describe the
ornaments.
Answer:
Ornaments were worn
by both men and women. Some of the common ornaments were
necklaces, finger -rings, bangles, armlets, anklets, nose
rings, fan shaped gead dress and earings. They were made
gold, silver, precious stones and ivory.
Question20.
Breifly describe the
dancing girl.
Answer.
The bronze statue of
a dancing girl, found at mohen jo daro, is a masterpiece of
art and it shows a high degree of development in art of
sculpture. The figurine shows vigour, variety and ingenuity.
The right arm of the dancing girl rests on the hip and the
left arm in heavily bangled. It holds a small bowl against
her left leg.
QUESTION21.
Mention the
evidences which suggest that the harappan peoplehad trdae
relations with other counteries.
Answer ;
1.
The mesopotamian records from about 2350
BC refer to trade relations with meluha(name given to
indians)
2.
objects of sumerian origin found at the
ruins of the indus cities indicate that their trade
relations between these countries were actively practised
into.
3.
The seal bearing a mastless ship holds
the evidence of popularity of the sea routs
NOTE: These include some
internal part also.
Chapter
2. Vedic Period
QUESTION1 name the categories of
vedic literature. Why was early vedic literature is known as
shruti?
Answer:
Vedic literature is
divided into two categories:
1.
Early vedic literature( Shruti)
2.
Later vedic literature (samriti)
It is believed that
four vedas and its parts( the brahmanas, the Aranyakas, the
upnishads) are given by god to sages by hearing(shruti means
which is heard) and so it is called Shruti.
QUESTION2.
Name the four vedas
and state what the hymns in each veda deal with?
Answer:
Four vedas:
1.
The Rig veda.
2.
The sama veda
3.
The Yajur veda
4.
The Atharva veda
1.The hymns of rig
veda dedicated by the sages to the gods. It contains the
famous gayatri mantra.
2. the hymns of sama
veda sung at the time of the sacrifice by the priests
3. the hymns of
Yajur veda deals with rituals perfomed at the time of
Yajnas.
5. the hymns of
Atharva veda deals with the power of ghosts, spirits, gyan,
karma and upasna.
QUESTION3.
What are upnishads?
Answer
The Upnishads are
philoshophical commentaries on the vedas. These form the
basic source of indian philoshophy. They are said to form
the foundation on which later additions to vedic literature
rest.
QUESTION4
What are
dharmashatras?
Answer
The law- books
called the dharmashatrasand the samritis together with their
commentaries are called dharmshastras.
Question5
What is known as
bhagwad gita?
Answer:
Bhagwad gita is the
compilation of the text, teachings imparted by lord
krishnato arjun explaining the importance of Karma or duty,
immortal soul, the right way of life. It also includes the
glimpse of mahabharat battle. It is a sacred book of hindus.
QUESTION6.
Mention the
importance of the Epics as a source of information about the
aryans.
Answer.
1.
these inform about the political, social
and cultural organisation of the epic age.
2.
these provide information about aryan
kingdom.
3.
These reveal the high ideals of family
life of the aryans.
QUESTION7
Which battle is
known as the mahabharata
Answer: the battle
between kurus and pandavas along the battlefield of
kurukshetra is known as the mahabharats.
Questiion 8
Give two features of
painted grey ware.
Answer:
The PGW is a very
fine, smooth and even-coloured pottery. It was made out of
well worked, high quality clay with geometric patterns
painted on it in black colour. Floral pattern and sun
symbols are seen in some cases. The pottery include open
mouthed bowls and dishes.
QUESTION9
How did trade become
a pivot(central point) around which the life of the people
revolved?
Answer:
During the later
vedic age, trade became quite prominent; so the settlement
or the towns and cities developed around the main trade
centres. Thus, the city life moved according to the
activities associated with the trade.
QUESTION10
What was the role of
iron?
Answer
Iron played a
significant role in the lives of peasants as the use iron
became popular for making agricultural tools. Iron
ploughshares and metal tools were used and a variety of
crops cultivated.
QUESTION11.
State the difference
in the position of women between the early vedic age and
later vedic age.
Answer:
During the early
vedic period wome were respected. The institution of
marriage had become sacred.
The daughters were
given freedom to choose their husbands.
In the later vedic
age, there was significant decline in the status of women.
Their participation in yajnas was not considered necessary.
They did not enjoy the right to property. Man’s opinion were
respected. As a result the freedom to choose the husbands by
women was curtailed.
QUESTION12
What are varnas?
Name them
Answer;
Varna was a division
on the basis of skin colour. In vedic age it became popular
as the society was divided into four varnas. Brahmins,
Kshatriya, vaishayas and shudras.
QUESTION13.
Name the four
ashrams into which the human life span was divided,
indicating the time span for each
Answer:
The four ashramas
are Brahmacharaya, Grihastha, Vanaprasthas and sanyasa
1.
Brahmacharya ashrama: it lasted upto the
age of 25 years. During this period, the pupil was expected
to acquire knowledge in the gurukul and observe strict
discipline.
2.
Grihastha ashrama: during this peiod, man
was supposed to marry and raise a family. This perios lasted
from the age of 25-50 years.
3.
Vanaparastha ashrama: it lasted from the
age of 50-75 years. This period man was expected to retire
from worldly life and acquire and philosophical knowledge.
4.
Sanyasa ashrams: the last stage lasts
from the age of 75 to 100 years this period of renunciation
QUESTION14.
State the change
that occured in the position of brahmins in the later vedic
age?
Answer:
In the later vedic
age brahmins or the priests performed religious rituals. The
caste system become rigid. The priests came to be considered
as gods on earth.
QUESTION15.
State three
important changes that took place in the society in the
process of its transition from early vedic period to the
later vedic period.
Answer:
Three changes:
1.
Single family converted to joint family.
2.
Equal rights enjoyed by women changed to
declination in status of women.
3.
General caste system converted to rigid
caste system.
Question16
Difference between
the economy of early vedic age and later vedic age.
Answer
Early vedic age:
In the beginning the
main occupation was domestication of animals and agriculture
was secondary.
Cattle was the
important source of wealth.
Many engaged in
trade and commerce. Dyeing, embroidery;carpentry, weaving,
pottery, crafts in gold and iron were important occupations.
Later vedic age:
Agriculture became
the chief accupation while domestication of animals also
continued.
Land was the
important source of wealth.
Traders guilds had come up and
trade had become very important. Besides occupation of
earlier period, many new occupations like physicians,
musicians, and many other profession emerged
.
Note
: these questions will include an inside portion.
Class 9th subject history and civics
Chapter 1 of civics
Question 1:
What is meant by the
term constitution
Answer:
The Constitution is
a comprehensive document containing the set of rules
according to which the government of a country runs. It
regulates the position and powers of three organs of the
government- the legislative, the executive and judiciary.
Question 2:
On the basis of
which plan was the constituent assembly constituted?
Answer:
Cabinet mission
plan.
Question 3
What is objective
resolution?
Answer:
Objective resolution
was proposed by pt. Jawahar lal nehru on december 13,
1946 for highlighting the national goals.
Question 4;
By whom was the
objective resolution proposed?
Answer:
It was proposed by
the Pt. Jawahar Lal Nehru.
Question 5:
Who was appointed as
the chairman of the drafting committee of the constitution?
Answer
Dr. Bhim Rao
Ambedkar was appointed as the chairman of the Drafting
committee.
Question 6:
When was the
constitution adopted and passed? When did it come into
force?
Answer
-
Adopted and
passed: 26 November 1949.
-
Come into force:
26 January 1950.
Question 7:
State the
significance of January 26.
Answer :
It was on this date,
January 26, in 1929, that the lahore session of the indian
national congress had for the first time given the call for
purna swaraj. Since then, the day was celebrated as
independence day upto 1947, but later on, it was designated
as the republic day.
Question 8:
What is the
preamble?
Answer :
The Preamble is the
introductory part of the constitution, which sets out the
main objectives of the constitution.
Question 9;
Explain the
significance of the term sovereign.
Answer:
It means that India
is its own supreme power and not a subject of any other
state or country.
Question 10.
Mention any two
features indicating the significance of the preamble.
Answer:
1.The preamble
represents the essence, the philosophy, the ideals of the
entire constitution of india.
2. It contains the
five basic features of the constitution ie. sovereign,
socialist, secular, democratic and republic.
Question 11.
Who represented the
anglo indians in the constituent assembly /
Answer
Mr. frank anthony
and S.H prater
Question 12.
Why is our
constitution known as the fundamental law of the land?
Answer
Being superior to
the ordinary law of the state, the constitution of India is
known as the fundamental law of land.
Structured questions
Question 1
With the reference
to the making of indian constitution explain the following
Part a.
When and
how……...elected?
Answer 1.
The first sitting of
the assembly was held on december 9, 1946
The cabinet mission
plan arrived in India in 1946 and put forward a set of
proposals to meet the demands of freedom fighters. One of
these proposals involved setting up of a constituent
assembly whose members were to be elected
indirectly by the provincial legislative
assemblies.
Part b.
How was
the membership……..partition of the country?
Answer
The muslim league
boycotted the constituent assembly consequently the members
representing the territories went
to pakistan withdrew from the cosntituent assembly of
india. As a result, the membership of the constituent
assembly of india stood at 299n against the original number
of 385.
Part. c how can you
say that constituent …………….of the indian society.
Answer:
The wide ranging
membership of the constituent assembly gave representation
to all shades of public opinion.it was composed of members
from all the categories and communities belonging to india.
Question 2
With the reference
to objective resolution
Part a.
Same as the 4th
answer. Indian idependence act 1947 gave legal sanctity to
the constituent assembly.
Part b
Main points
Answer1.
-
India will be a
republic.
-
The ideals of
social, political and economic democracy would be
guaranteed to all the people.
-
The republic
would grant fundamental rights to citizens.
-
The state would
safeguard the rights of backward and minorities.
Part c.
Three principles
Answer:
-
Making indian
constitution workable, flexible and strong enough to
hold the country together both in peace and in war.
-
Providing special
safeguard to the minorities.
-
Incorporating the
right to constitutional remedies.
Note: these questions cover the inside portion also.
9th Class Chapter 3rd Jainism and Buddhism
Question 1 angas and subject matter
Answer
Jain scriptures are popularly known as agamas i.e what comes
out from the mouth of the lord. The teaching of lord
mahavira compiled by his disciples in 12 parts called angas.
These deal with code of conduct for jain monk, and also
expound the jain doctrines in a comprehensive manner.
Question 2.
Triptikas…?
Answer:
The triptikas are the most important literary works of the
buddhists. The word triptikas means three baskets of
buddhists canon.
name :
3.
The vinaya pitaka.
4.
The suta pitaka.
5.
The abhidhamma pitaka.
Question 3. Causes … rise.
Answer:
5.
The rigid caste system.
6.
Monopoly of priest for expensive rituals.
7.
Difficulty of the common people to understand Sanskrit
language.
8.
Role of kshatriya princes.
Question 4.
Who
was………. His name?
Answer:
Vardhaman Mahavira was the 24th and the last of the
tirthankaras.
His father held a prosperous kingdom at his birth time, so
he was called vardhamana which meant prosperity.
Question 5.
Five vows….
Answer.
4.
Ahimsa - non violence.
5.
Asateya - not to steal.
6.
Satya - not to tell a lie.
7.
Aparigraha - not to possess propoerty.
8.
Brahamcharya- to practise chastity.
Question 6.
Tri ratnas.
Answer: according to mahavira, there were three ways to
attain moksha, known as tri ratnas ie.
1.
Right faith.
2.
Right knowledge
3.
Right conduct.
Question 7.
Causes…. Jainism.
answer:
1.
The use of simple dialect that is prakrit for preaching.
2.
The solid patronage by great kings.
Question 8
Two sects…..
Answer:
The two sects of jainism were as follows:
1.
Shvetambaras.
2.
digambaras.
Question 9
Gautam buddha… born?
Answer:
Gautam buddha was the founder of buddhism. He belonged to
the kashatriya clan of sakya. He was born in 563 BC at
lumbini near kapilavastu.
Question 10.
What are ……...sights?
Answer:
1.
An old man bent with age
2.
A sick man groaning with pain.
3.
A dead body of man being carried .
4.
An ascetic in search of salvation.
Question 11.
When and ……….. Enlightenment?
Answer:
After leaving home in search of truth, gautama wandered from
place to place. Afterwards he studied under renowned
teachers of rajgriha and at last he went to gaya and
practised severe penance and life of austerity and finally
at the age of thirty five was enlightened sitting under a
peepal tree that place is known as bodh
gaya in bihar.
Question 12.
Noble truths ….
Answer:
1.
The world is full of suffering.
2.
The suffering has a cause.
3.
Desire is the cause of suffering
4.
If desire is stopped, suffering can also be stopped.
Question 13.
Four points……….buddha.
Answer:
1.
Right action
2.
Right thought
3.
Right belief
4.
Right living.
Question 14
Meaning ...name
Answer:
Buddha: gautama after attaining knowledge came to
be known as buddha means the enlightened one.
Tathagat: buddha is also called tathagat means the founder
of the truth.
Question 15.
Two principles……..sangha.
Answer:
1.
To speak the truth.
2.
To abide by brahmacharya.
Question 16.
Features of buddhism…
Answer:
1.
Buddha inspired the people to lead a simple life.
2.
He used simple language.
3.
Easy to follow no complex rituals.
Question 17.
difference …. Hinyana and mahayana.
Answer:
|
Hinyana
|
Mahayana
|
|
Buddha was
regarded as human being who attained enlightenment
through own efforts
|
Buddha was
regarded as the incarnation of god and provider of
salvation
|
|
It shuns
idol- worship, buddha was not believed as god
|
The image of
buddha was worshipped
|
Question
18.
Give ….causes of ….
Answer:
1.
Division of Buddhism into two sects.
2.
Corruption in sungha.
3.
Monks and nuns started living worldly lives.
Class
9 Geography
CHAPTER-ROCK STRUCTURE [4]
Ans 1:Lithosphere is the solid crust of rocks on the surface
of the earth on which we live.
Ans 2: The continental layer and oceanic layer are the two
layers of the lithosphere.
Ans 3: Mantle lies below the crust. Its exact position is
between the lithosphere and the core of the earth.This layer
is also known as Mesosphere.
Ans 4: Mantle is the second layer of the earth between the
crust and the core with its upper boundary marked by
Mohorovicic Discountinuity and its lower boundary marked by
the Gutenburg Discountinuity.
Ans 5:Core is the central part of earth below a depth of
2900 km. It is also known as Barysphere.
Ans 6: Sial layer is composed of silica [Si] and aluminium
[Al]. Therefore it is known as the layer of sial.
Ans 7: PROPERTIES OF CORE OF THE EARTH:
[1]Core the inner most layer of the earth.
[2] It is composed mainly of iron and nickel.
[3]It is the thickest part of the earth and divided into two
parts outer core and inner core.
Ans 8 earth is composed of three parts crust , mental and
core.
Ans 9 Core is composed of iron and nickel and is called
nife.
Ans 10 The temperature increase as we go down due to the gas
and pressure present inside the surface of the earth.
Ans 11 The difference between sial and sima.
SIAL [1]Upper most layer of the earth crust.
[2]Its thickness is nearly 20km.
[3]It is composed of silica and aluminium.
SIMA [1]Lower part of the crust.
[2]Its average thickness is about 25 to 30 km.
[3] It is composed of silica and magnesium.
Ans 12 : The two most important and abundant chemical
elements present in the earth crust are oxygen 46% and
silicon 28%.
Ans 13 : The contacts zone of the crust and mantle is called
Mohorovicic Discontinuity.
Ans 14 : Mantle is composed of magnesium and iron silicates
along with substantial quantities of sulphites in the upper
mantle and nickel and iron in the lower mantle.
Q-DEFINES THE FOLLOWING TERMS:-
[1] Crust-Earths outer shell of crystalline surface rock,
ranging from 5-60 km in thickness from oceanic crust to
mountain ranges.
[2] Mantle- It is a layer of silicate rock between the crust
and the outer core.
[3] Core- The central part of the earth below a depth of
2900 km .
[4] Nife- Core is composed of iron and nickel and is called
nife.
CHAPTER- 3
Rotation and Revalution
Ans no.1= The earth is tilted on Its axis. This tilting of
the earth on its axis is know as the inclination of the
earth axis .
Ans no.2= Coriolis Force.
Ans
no.3 = .The rotation of the Earth from west to east
determines the direction in which the sun moon and stars
appear to rise and set.
.Another proof of rotation of
earh is that if one drops a stone from top of a multistorey
building it will fall slightly towards the east.
Ans
no.4 = Centrifugal force causes slight flattening of the
earth at two polls .
Ans no.5 = The spinning of the
earth on its polar axis is termed as rotation.
. Effect
of Rotation
. The daily occurrences of the day and
night.
. The apparent movement of the celestial bodies.
Ans no.6 = Meaning of the term solstice and Equinoxes
solstice : An event occurring when the sun appears to
reach its most northerly or southerly excursion relative to
the celestial equator on the celestial sphere.
.
Equinoxes : The time when the sun crosses the plane of the
earths equator makng night and day of equal length all over
the earth.
Ans no .7 = Rotation causes the formation
of day and night . [Diagram on page no. 33 figure 3.2].
Ans no . 8 = Summer solstice on june 21, winter sostice
on december 22 After the spring equinox the sun appears to
move north and is directly overhead tropic of cancer on june
21 this is term as summer solstice
. On dec 22 the sun
shines overhead on tropic of capricorn in southern
hemisphere. The northern hemisphere has its winter solstices
.
Ans no .9 = Dec 22 is the longest day in the
southern
hemisphere .
Ans no. 10 = June 21 is the longest day in the northern
hemisphere.
Ans no. 11 = Orbit is the path along which the earth travels
when moving around the sun in 365 ¼ days.
Ans no. 12 = The arctic circle experience mid night sun on
june 21.
Ans no. 13 = Earth compete one circle around the sun 365 ¼
days. Every four years the extra one-fourth day is taken as
one whole day.
Ans no. 14 = Days and nights of equal duration on the
equator on 21 march and 23 september.
Ans no . 15 = If axix was perpendicular to the place of
ecliptic, every place on the earth surface
would have had 12hrs of day and 12hrs of night and
there would have been no seasons. [Draw figure no 3.5]
Ans no. 16 = The
period of rotation or the time required for the earth to
turn through 360 degree is 23hrs , 56minutes ,4.09sec . This
period is termed as sidereal day.
Ans no. 17 = The earth is said to be in perihelion on
January 3rd when distance least or about
147million km
Ans no.18= The speed of earth revolution varies according to
the path of the orbit occupied .The velocity is greatest at
perihelion and least at aphelion
Ans no. 19= The effect of speed of earth rotation are-
.The daily occurrences ofday and night.
.The apparent movement of the celestial bodies
Ans no.20= The two features of summer solstice are –
.The duration of day is longer than night.
.The length of day increases with increasing latitude north
of the equator.
Ans no .21= The two motions of the earth are rotation and
revolution
Ans no.22= The path along which the earth travel when moving
round the sun.
Ans no. 23= The earth rotates on an imaginary line called
axis but it is not at right angle to the plane of revolution
it make an of 66 1/2degree with the plane of ecliptic andis
tilted 23 1/2degree from a line perpendicular to that palne.
Ans no.24= SAME AS ANSWER NO 3.
Ans no.25=The time when the sun crosses the plane of the
earth equator making night and day of equal length all over
the earth.It occurs on march 21 and September 23.
PHYSICAL EDUCATION (9th Class)
CHAPTER-1
Very short answers:
1.
:-Support
:-Shape
:-Protection
:-Junction
:-Storehouse
:-Manufacture of RBC
(With explaination)
2. 206 bones. Longest bones Femur (present in thigh) and smallest bone
Stapes(present in ears)
3. Page no. 15(Down Right))
4. Page no. 12(Middle Right)
5.
:-Ball and Socket joint(at Shoulder and Hip joint)
:-Hinge joint (at Elbow joint)
:-Pivot joint (at Neck joint)
:-Gliding joint(at Carpals joint)
:-Saddle joint (at Thumb joint)
Match the following:
Sequence: 4, 6, 7, 2, 8,
1, 5, 3, 9
Short answers:
1. Done
2. Done
3. Femur,Tibia,Fibula, Tarsals, Metatarsals and Phalanges
4. Page no. 13(Left side)
5.
:-Long bones
:-Short bones
:-Flat bones
:-Irregular bones
:-Sesamoid bones
:-Cartilaginuous bones
(With explaination)
6. Three types:
:-Immovable or Fibrous joint
:-Slightly Movable or Cartilaginous joint
:-Freely Movable or Synovial joint
(With explaination)
Long answers:
1.
:-Stronger bones
:-Reduces bone injuries
:-Improves ossification
:-More blood supply
:-Faster healing
:-Increases RBC
(With explaination)
2. Done
3. Bone(same diagram)
Cartilage (Cartilages are elastic hard connective
tissues. The elasticity is due to the presence of chondrin protein and some in
organic salts. Cartilage has lacunae and chondroblasts in its structure. It is
of three types i.e Hyaline, Fibrous and Calcified Cartilage.
4. Done
CHAPTER-2
Very short answers:
1.
:- Provide proper shape and structure
:- Provide effort
:- Bring external movement
:-Help in fluid movement
:- Forceful actions
:- Provide protection
(With explaination)
2. Trapezius, Levator Scapulae and Deltoid
3.
:- Skeletal Muscles
:- Smooth Muscles
:- Cardiac Muscles
(With explaination)
4. Page no. 22
5.
:- Isometric exercises
:- Isotonic exercises (Concentric and Eccentric exercises)
:- Isokinetic exercises
(With explaination)
Match the following:
Sequence:
2, 7, 4, 1, 3, 8, 6, 5, 9
short answers:
1. Done
2. Each muscle is made up of thousands of long and narrow muscle cells
called muscle fibres. These muscle fibres are arranged in bundles and enclosed
within a tough layer of connective tissue called Epimysium(Sarcolemma).
Every muscle fibres is made up of a very large number of microscopic
threads called Myofibrils. Each Myofibril consists of rows of minute protein
molecules (Actin and Myosin). It is shaped like two combs with their teeth
interlocked at the ends.
3. Page no. 24(any six with their functions)
4.
Isometric Exercises (same length of muscles,no visible movement of
muscles,no external movements, work done is zero)
Isotonic Exercises (same tension in muscles, visible movement of muscles,
external movement, work is done during these exercises)
5. Done
Long answers:
1.
:- Gain in strength
:- Efficiency improvement
:- Lactic acid tolerance
:- Proper shape of body
:- Faster recovery from injury
:- Increase in muscle mass
2. Done
3. Done in short answers
4. Done
5. Done
FOOTBALL(2nd Term)
1.
(a)It is the most common skill used to hit the ball with power. It
reaches long away to the desired position. It is performed in various ways
like kicking with inside or outside of foot, instep kick,punt kick, scissor
kick, banana or chip kick,roll back kick etc. It can be used during free kick,
goal kick, corner kick, penalty kick or when situation suits.
(b) Usually an attacker heads the ball to redirect it towards the net. A
defender heads the ball to deflect it away from the goal. In this forehead
hits the overhead coming ball.
(c) This skill is used to throw the ball back for the play from the side
or touch line. The player must throw the ball over the head while both the
feet should be in contact with ground. The player must not touch the sideline
during throw in.
(d) During a knock-out tournament, it there is a tie at the end of
regulation time then the teams play for 30 minutes extra time of two half of
15 minutes each overtime period. If there is a tie after the overtime period,
a penalty shoot-out takes place. Each team chooses 5 players to take the kicks
in turns. If the teams are still tied after 5 kicks each, the team keep taking
penalty kicks until one team wins. This procedure is called Sudden-Death.
(e)During a knock-out tournament, it there is a tie at the end of
regulation time then the teams play for 30 minutes extra time of two half of
15 minutes each overtime period. In this duration if any team makes the goal,
wins the match. Earlier there was golden goal period in which the team who
scores the goal first wins the game but now it is said as silver goal period.
(f)It is given when referee shows the warning card to the player. In this
foul has been committed outside the penalty area like intentional hit to the
player, intentional handling the ball, charging, dangerous play, holding
opponent from behind, violent play, kicking the opponent. While taking
direct free kick the opposing player should be at least 10 yards away from the
ball. Goal can be scored from direct free kick.
(g)It is also known as flag kick. Corner kick is awarded when a defender
puts the ball out of the play behind his team's goal line. An attacking player
then tries to send the ball in front of the goal for another attacker to head
or make a short pass to a teammate to convert it into goal. It is taken from
corner arc or quarter yard circle.
(h)A penalty kick is awarded when a foul is committed by a defender in
the penalty area. The ball is placed on the penalty spot and the attacking
player tries to kick it directly into the goal and goalkeeper only defends it.
The offences (fouls) are like intentional hit to the player, intentional
handling the ball, charging opponent, holding opponent from behind etc.
(i) Player is expelled if he commits a serious foul like strikes or
charges or kicks or attempts to kick it trips on opponent, holds opponent,
handles the ball intentionally, uses abusive or offensive or insulting
language, receives a second yellow card during the game.
(j)A pass between defenders into the open space between the fullbacks and
the goalkeeper with the idea that a forward will beat the defenders to the
ball. There are two types : a Straight Through Pass and a Diagonal Through
Pass.
(k) Done (same as free kick)
(l) Corner arc is also known as quarter yard circle from where corner
kick is taken. It is on all four corners. The dimension is 1 yard(91cms)
(m)It is starting the game (in beginning or after half-time or after the
goal has been scored or in extra time). During kick-off players remain in
their own half. Opponent team player doesn't enter 10 yards circle until ball
is pushed or kicked forward. Kick-off player should not touch the ball
consecutively second time until played by another player.
(n) The time which game has been stopped temporarily due to injury of the
player. This stopped duration of game is added in regular time after each
half.
(o)A kick in which the ball is placed on the ground before kicking eg.
Penalty kick, Corner kick, Kick-off etc.
(p)The penalty area(outer box) is 18 yards deep, so the ball is only 6
yards from the top of the penalty area. Therefore, the arc(outer circle) is
drawn so that all players must be 10 yards away from the ball at the taking of
a penalty kick. Also an arc with 9.15m radius drawn outside the penalty area
box.
(q) Federation International de Football Association, formed in 1904.
2. Draw Football playfield with all the dimensions from book.
Positions:
Left forward-Center Forward-Right Forward
Left Mid Fielder-Center Mid Fielder-Right Mid Fielder
Left Full Back-Center Full Back-Right Full Back
Goalkeeper
3.
Goalpost (8yard * 8feet with 5 inches thickness of post)
Goal Area(6yards * 20 yards)
4. Done
5. 30 minutes extra time, divided into two halves of 15-15 minutes
each.
6.
Direct Free kick
Indirect free kick
Corner kick
Penalty kick
Kick-off
(With explaination)
7. In India, the Britishers brought this game and formed Football
Association in 1878. Football became very popular in Bengal. Many clubs were
established to improve Football from Bengal. India had started the second
oldest Football tournament known as Durand Cup in 1888 from Shimla. Later,
this venue was changed to New Delhi from 1940. There are many clubs associated
with Football in India like Mohan Bagan, Mohammedan Sporting, East Bengal,
JCT, Salgaokar, Mahindra and Mahindra etc.
FIFA(Federation International de Football Association) is the controlling
body at International level.
8. Many Football clubs are formed for participation in European league,
which had hightened the Football standards in Europe. The best Football
players of the world play in European league from different Football clubs
like Real Madrid, Barcelona, Juventus, Arsenal etc.
9. Player's basic equipments (Jersey with number,Shorts,Long Stockings,
Studs, Shin Guard, Gloves for Goalkeeper etc.)
Football specifications (27-28 inches circumference, 14-16 ounce weight,
Leather or Rubber or Synthetic made). The air pressure equal to 0.6-1.1
atmosphere.
10. A player is offside when he is close to the opposing goal line
without the ball, unless two defenders are between the attacker and the goal
line. Offside is given by linesmen by raising red flag. Goal is not considered
in that case and opponent is awarded with indirect kick.
11.
Yellow Card fouls (not respecting referees decision, delay the game,
argument, unsportsmanlike conduct, if goalkeeper keep the possession of the
ball for more than 6 seconds)
Red Card Fouls (strikes or charges or kicks or trips or holds the
opponent, handles the ball intentionally, uses abusive or offensive or
insulting language)
Fouls are regulated by Match Referee and two Linesmen.
12. When the ball completely crosses the goal line, either in air or
grounded, between the goalposts and under the crossbar of goalpost. It must be
scored in a fair manner.
13. Any six(Name and Country)
14. Done
15.
Three substitutions
Substitution procedure is already discussed.
16.
Match Referee regulates the time record of game.
Injury Time (The time for which game has been stopped temporarily due to
injury of the player. This stopped duration of the game is added in regular
time after each half.
17. Any three(with their name and year)
18. Syed Naeemuddin
19. During a knock-out tournament, it there is a tie at the end of
regulation time then the teams play for 30 minutes extra time of two half of
15 minutes each overtime period. If there is a tie after the overtime period,
a penalty shoot-out takes place. Each team chooses 5 players to take the kicks
in turns. If the teams are still tied after 5 kicks each, the team keep taking
penalty kicks until one team wins. This procedure is called Sudden-Death.
20.
Yellow Card fouls (not respecting referees decision, delay the game,
argument, unsportsmanlike conduct, if goalkeeper keep the possession of the
ball for more than 6 seconds). Yellow Card is a warning card.
Red Card Fouls (strikes or charges or kicks or trips or holds the
opponent, handles the ball intentionally, uses abusive or offensive or
insulting language). Red card is an expulsion card. To whom it is shown can
not play further in the same match. Also,under such condition substitution can
not be done.
21.
-Unsupportive Behaviour
-Abusive words or action
-Delays the restart of game
-Enters in playfield without permission of Referee
-Leaves playfield without permission of Referee
-Infringes the laws of game
22. Red Card
23. Yellow Card
24.
Fouls (kicking or Trips or Holds an opponent)
Offences (Deliberately Delays game, infringement of rules, argumentative
behaviour)
25.
Trapping the ball by Chest,Thigh and under the feet.
26.
Dropped Ball(In case of struggle for ball possession when both players
commit simultaneously foul, in that case the Referee stops the game for some
time and afterwards drops the common ball to get the possession of the ball.
Play restarts when ball touches the ground.
Heading (An attacker heads the ball to redirect it towards the net. A
defender heads the ball to deflect it away from the goal. In this forehead
hits the overhead coming ball.
27. Done
28. Done
29. Done
30.
-The Ball(at penalty spot, i.e 11m from goal line)
-The Defending Goalkeeper (In front of goal line,in own goal area)
The Players (Behind the outer circle, i.e arc drawn outside the penalty
area box)
31. Done
32.
(a)110m and 75m
(b)90 minutes for men
80 minutes for women
Extra time (30 minutes divided into two halves of 15-15 minutes each)
(c)
8 yards and 8 feet
(d)
10 yards and 1 yard
VOLLEYBALL
1.
(a)A specialized defensive player who plays in rear half to provide rest
to certain Player. He can be substituted anytime during match from rear row
player. He wears different colour kit. He can not serve, block and smash (he
can smash behind the attack line)
(b)After every change of service, the players of serving side rotate in
clockwise direction, otherwise, it may be a foul.
(c)In Tennis Service, the server can stand anywhere behind the 9m see
line and executes it as a spike behind the service line. If it is hit well,
the serve is a powerful offensive skill.
(d) It is a powerful attack. It has variations like back court spike,
line shot, cross court shot, dip, block abuse, off-speed hit, slide, double
quick hit etc. Player who executes the spike have an excellent sense of
balance in the air and can perceive and anticipate the actions and positions
of the opposing team members. Contact with the ball takes place above the net.
A player specialized in smash is known as spiker.
(e)It is a rally technique used during the match. The tip is a transitory
move between reception and attack like overhead set. It is made with an upward
movement of the arms and legs. Contact with the ball is with the fingertips
without touching palm. A player specialized in lifting is known as Booster or
Setter.
(f)Bump is a defensive skill keeping the ball from falling, receiving
serve and offensive skill as it provides good chance to tip the ball for the
spiker. Bump is the first contact of the ball (service reception). The bump
provides the transition between defence and attack.
(g)It may be executed by one, two or three players and it is the first
line of defence against the spike. Blockers are not expected to block all
spikes but to do as a screen, reducing the floor space that must be covered by
the players behind them. To block, a player has good anticipation of the play.
The tallest players are usually the main blockers.
(h)A ball hit into the net, by a team may still be kept in play (up to 3
hits) provided that the net is not touched by a player. Players may not touch
the net. If 2 opposing players touch the net simultaneously, the ball is
declared dead and is replayed. Also when the ball touches the hand of blocking
players during smash and fall outside the court.
(i)A player specialized to hit the ball down towards the opponent's
court. He is also known as smasher.
(j)A player specialized to lift the ball for the smash. It is performed
over the coming ball from own teammates. This player is also known as Booster.
(k) During service the players must stay in their positions i.e
diagonally opposite player must be in the same manner as in beginning.
Otherwise, positional fault is given to opponent.
(l)A hit aimed at opponent's court as to gain a point and a service in
form of spike,dig,dink etc.
(m)A player of servicing team must not prevent the visibility of service
player (server). The collective screening, from seeing the server or the
flight path of the ball is illegal.
(n)An extended upright over the net which gives the idea of sideline. It
is 180cm in length whereas 80cm above the net. It is made up of fibreglass,
flexible in nature.
(o)An extended line behind the center line which restricts libero to
smash from front area of court. It is 3m away from the net.
(p)An area behind the backline which is used for performing service. It
extends 3-5m behind the service line.
(q)It is given for 60 seconds in the final set when leading team reaches
the 8th and 16th points. It may be requested by each team.
(r)It is temporarily interruption asked by the coach during game. Each
team may request a maximum of two timeouts. All requested timeouts last for 30
seconds.
(s)A fault in which a wrong positional player performs the service.
(t)A fault given when the opponent player enters into the opponent area
in air, by crossing center line by foot or by hands over the net during smash,
block or lift.
(u)A player when takes the support from teammate or any other structure
or object in order to reach the ball within the playing area.
2. The game of Volleyball, originally called "Mintonette" was invented in
1895 by William.G.Morgan after the invention of Basketball. Volleyball rules
were framed in 1928 by United States Volleyball Association (USVBA). IN 1947,
the Federation International de Volleyball (FIVB) was founded in Paris. It was
included in the 1964 Olympic Games. In India, Volleyball came into Vogue
through YMCA and later Volleyball Federation of India (VFI) was formed in
1950.
3.
:- One team have total 12 players (6 playing and 6 substitutes)
:- The team that wins a rally, wins a point and a service.
:- A set is won by scoring 25 points.
4.
Net(size of net : 9.5m * 1m width, height of net : 2.43m for men and
2.24m for women)
Ball(circumference of ball : 65-67cm, weight of ball : 270gm)
Antenna(Done)
5. Replacement of one or more players from the listed substitution apart
from libero. The coach requests for substitution to the assistant referee. An
area 3m away towards sideline is allowed to move for substitution. When
referee signals for substitution the player should move out and substitute
player should enter. There are two types of substitutions, legal and illegal
substitution.
6. There are lots of variations of service like underhand,top spin,
floating,jump,jump float, round arm, tennis service etc.
7.
Lift(Done in question 1 (e) part)
Bump(Done in question 1 (f) part)
8. The advantage of block is to stop the spike/smash,so that ball should
fall in opponent court and to gain a point and a service.
It is performed by front row tall player/players, either one,two or all
three players together. They takes vertical jump with their arms stretched up
in the air and open palms on the way of spike trajectory.
:- Net touch
:- over the net penetration
:- under the net penetration
:- body contact with opponent
:- if a back row player blocks
:- if libero blocks
9. When there is a tie at 24-24 points each, the game is extended for two
points because to win a set one team should have a lead of 2 points.
10. From book with all dimensions
11. After every change in service, the players of the serving side rotate
in clockwise direction so that each player should get equal chance to play in
front and back zone both except Libero.
Positional fault (Done)
12.
Attack line(Done)
Back line (A line at the back of court at 9m from the net. It is also
known as service line.
13.A free zone area on both the sides of court, which extends up to 3m,
where player of both the teams performs warming-up for 3-5 minutes before the
start of the match.
14. 3 contacts
15.
Set(A set is won by a score of 25 points. And if there is a tie at 24
points then game continues until one team has a two points lead)
Match(A match is won by winning 3 sets out of 5)
16. Done
17.
Legal substitution (same as substitution-already discussed)
Illegal substitution (Each team can do 6 substitutions in a set. In case,
if there is injury or expulsion and team has already completed their 6
substitutions in a set, under such condition the substitution done is known as
illegal substitution. A team who ask for it, is penalized. A point and a
service is awarded to the opposing team.
18. Done
19.
First Referee (Regulates the rules, starts and ends the match,
Whistles,gives violations, fouls and penalties)
Assistant Referee (Gives rotation fault, positional fault and line cut)
Captain (Performs toss, give positions to players, ask time outs and
substitutions from Referee, with the consent of coach)
20. Any six
21. Beach volleyball is played on a Sandy surface. Only two players on
either side plays at once. FIVB added it in world championship series in 1987
and later in 1996 it was added to the Olympics.
22. Done
23. Rotation fault, penetration fault, positional fault,net touch,
service line cut during service and 8 seconds service delay violation.
24. Time outs, Intervals after each set, Objections, Instructions by
officials, legal substitution, illegal substitution, ball out of play etc.
Also due to violations,fouls, exchange of words between players, physical
fights,Bad or poor lights, equipments failure,poor playing surface,team
arrives late at venue etc.
25.
Penalty (Yellow Card) : deliberately delaying of the game, provoking
opponent by words or actions etc.
Expulsion (Red Card) : Use of abusive language, Physical fight etc.
Disqualification (Red and Yellow Cards jointly) : Shouting on referee
from bench area, disturbing table & court officials etc.
26. If match is of league tournament then equal points will be shared by
both the teams. And if match is of knock-out tournament then match can be
restarted after interruption if possible. Else, match can be resumed from the
same points on the kept spare day. Otherwise can be played from beginning on
some other selected day.
27.
Sanctions imposed by Referee:
Warning : Verbal warning,
Penalty : Yellow Card,
Expulsion : Red Card,
Disqualification : Red and Yellow Cards jointly.
28. Net fault (when ball stuck in net during service), service line cut
by server, screening, ball lands out of bounds, Rotation fault and Positional
fault.
29. Done(24)
30. Done(26)
31. Signals out(when ball lands out of court), signals in(when ball lands
in the court), judgement of side with the help of the Antenna.
32. Done
33. Two white side bands are fastened vertically to the net and placed
directly above each sideline. They are 5cm wide and 1m long and are considered
as part of the net. They are elastic in nature.
BADMINTON
1. (a)It is delivery of the shuttle to opponent court to begin with
rally.
(b) This is an offensive as well as defensive stroke. It is taken with
overhead swing of the racket to hit the shuttle hard so that it goes deep and
back in the opponent's court. It may be high clear or low clear, over the both
sides of the court in opponent's side.
(c)This is an offensive stroke to stop the rally at once. In this,
shuttle is powerfully hit in downward direction and gives no chance to the
opponent to return. Shuttle is hit with very fast swing of hand just over the
head. Shuttle goes steep down in opponent's court.
(d) During service both the feet should be stationary, otherwise, it is a
fault.
(e)It is the way of scoring in the beginning i.e the score is zero at
both sides.
(f)A game(setting) consists of 21 points continuous scoring, in the best
of three games(sets).
(g)An area between the back boundary lines and long service line for
doubles. It is the back area of the court where long service is considered out
during doubles game, also known as Back Alley.
(h)It is the continuous return of strokes by both the players over the
net.
(i)It is the last point in the game by which game winner is decided.
(j)It is playing the strokes from the left side of the body (for right
handed player).
(k)It is the redelivery of the same point. It is called by umpire due to
unforeseen situations like : umpire is unable to see the landing of shuttle,
fault at both sides of the players simultaneously, if receiver is not ready,
shuttle breaks during rally etc.
(l) During service the shuttle has to be delivered below the waist level,
otherwise, it is a fault.
(m)A variation of forehand stroke in which shuttle is hit hard but at low
height.
(n)A shot in which shuttle crosses the net in a diagonal direction.
(o) When score is at 20 all(20 each) on both sides.
2. The modern version of Badminton is said to have its origins in the
city of Pune in India and was initially called 'Poona'. British Army officers
posted there were the first pioneers of the game who took it to Europe. The
game was played in 1873 in a place called 'Badminton House' in England, from
where it got its name.
3. Draw Badminton court and give all dimensions from book.
4. 5 feet at the center and 5 feet 1 inch near the poles.
5. Player/Team, whoever wins the rally, is awarded with a point and a
service. Also, in case of fault done by one player, a point and a service is
awarded to the other player.
6.
:- A game consists of 21 points.
:- Continuous point scoring is followed.
:- Change of sides are done after each game and also on the 11th point in
the final game.
7.
:- Clear(explain)
:- Smash (explain)
:- Drive (explain)
Already done in question(1)
8. In case of tie at 20th point, the difference of two points will be
considered for deciding game.
9. Service is performed diagonally i.e. from right to right and left to
left side over the net as to begin the rally.
Service is illegal, if it is delivered from above the waist level, both
feet are not stationary at the time of service, delivered from wrong side of
the court and wrong player delivers the service.
10. In doubles game a team of two players each plays against the other.
They begin the game with the service from right side of the court to right
side opponent player(diagonally). A game consists of 21 continuous points. A
team who wins a rally is awarded with a point and a service. After winning a
rally, teammates changes their sides i.e. right side player goes to left side
and left side player to right side(opponents will not change their positions,
until they wins a point on their service). Same player serves as long as he is
winning continuous points.
11.
Drop(An offensive stroke hit from deep end which lands close to net)
Smash (Done)
12. Done
13. To check condition of playing surface, conducting toss, to call out
score after end of each rally, to check flight of shuttle in case of dispute,
to give break for cleaning sweat and to sit on high bench to observe the match
carefully.
14. To check waist fault, foot fault, line cut, service court error,
changes shuttle on demand of player, cleans sweat in case falling over the
court and sits opposite to Umpire at normal height.
15. arming-up exercises (shuttle run, squats, sit ups, push ups,
stretching, skipping, zig-zag running etc.)
Conditioning exercises (lunges, s
CLASS
9 BIOLOGY
Chapter 2 - Cell: The Unit Of Life
Question 1
1. Which one of the following cell organelles is
correctly matched with its function?
(a) Ribosomes
Synthesis of proteins
(b) Mitochondria
Secretion of enzymes
(c) Plasma membrane
Freely permeable
(d) Centrosome
Carries genes
2. All life starts as
(a) an egg
(b) a single cell
(c) a gene
(d) a chromosome
3. Which one of the following is found both in the
cells of a mango plant and a monkey?
(a) chloroplasts
(b) centrioles
(c) cell wall
(d) cell membrane
4.
A plant cell can be identified from an animal cell by the
(a) absence of centrosome
(b) presence of cell membrane
(c) presence of vacuoles
(d) none of the above
5. Plant cell has a cell wall made of
(a) Protein
(b) Fructose
(c) Cellulose
(d) Fatty acids
6. The cell organelle that helps in respiration of the cell is
(a) Mitochondria
(b) Lysosome
(c) Ribosome
(d) Centrosome
SOLUTION 1
1.
(a) Ribosomes
Synthesis of proteins
2. (b) a single cell
3. (d) cell membrane
4. (a) absence of centrosome
5. (c) Cellulose
6. (a) Mitochondria
Question 2
Name the part of the cell concerned with the
following.
(a) Liberation of energy
(b) Synthesis of proteins
(c) Transmission of
hereditary characters from parents to offspring
(d) Initiation of cell
division
(e) Hydrolytic in
function
(f) Entry of only certain
substances into and out of the cell
SOLUTION 2
(a)
Mitochondria
(b) Ribosomes
(c) Chromosomes
(d) Centrosome
(e) Lysosomes
(f) Cell membrane
Question 3
State whether the following statements are true (T) or false (F):
(a) All animal cells contain a cell wall. T/F
(b) A cell wall is made up of protein. T/F
(c) Centrosome occurs in animal cells. T/F
(d) Plant cells contain large vacuoles. T/F
(e) Protoplasm is the part of the cell which surrounds the
nucelus. T/F
(f) Genes are located in chromosomes. T/F
(g) Anthocyanins are the pigments of flowers, which are
dissolved in cell-sap. T/F
SOLUTION 3
(a)
F (False). Animal cells do not contain a cell wall.
(b) F (False). A cell wall is made up of cellulose.
(c) T (True)
(d) T (True)
(e) F (False). In eukaryotes, cytoplasm is the part of the cell which
surrounds the nucleus.
(f) T (True)
(g) T (True)
Question 4
How many chromosome pairs are found in human cells?
SOLUTION 4
23 pairs of chromosomes are found in human cells.
Question 5
What is the name of the chemical substance which constitutes the genes?
SOLUTION 5
DNA (Deoxyribonucleic acid)
Question 6
Match
the items in column 'A' with those in column 'B'
|
Column
A
|
Column
B
|
|
(a) Vacuoles
|
(i) Intracellular
digestion
|
|
(b) Nucleolus
|
(ii) Respiratory
enzymes
|
|
(c) Lysosomes
|
(iii) Covered by
tonoplast
|
|
(d) Anthocyanin
|
(iv) Dissolved in
the cytoplasm
|
|
(e) Cristae
|
(v) Forms RNA
|
SOLUTION 6
|
Column
A
|
Column
B
|
|
(a) Vacuoles
|
(iii) Covered by tonoplast
|
|
(b) Nucleolus
|
(v) Forms RNA
|
|
(c) Lysosomes
|
(i) Intracellular
digestion
|
|
(d) Anthocyanin
|
(iv) Dissolved in
the cytoplasm
|
|
(e) Cristae
|
(ii) Respiratory
enzymes
|
Question 7
Fill in the blanks:
(a) _________ consists of membranous sacs and secretes 40 types of digestive
enzymes.
(b) _________ is surrounded by microtubules, located near the nucleus.
(c) Very thin flexible, living membrane which is differentially permeable, is
called ___________.
(d) More than 1000 chromosomes are found in the nucleus of certain
(e) _________ are hereditary units.
(f) _________ is a plastid which stores starch.
SOLUTION 7
(a)
Lysosome
(b) Centriole
(c) Plasma membrane
(d) Insects
(e) Genes
(f) Leucoplast
Question 8
It is said that the protoplasm cannot be analysed chemically. Why?
SOLUTION 8
Protoplasm is the living matter of the cell. Protoplasm cannot be analysed
chemically because the chemical composition of protoplasm is very complex. It
varies slightly from one cell to another, although the common elements
included in the composition of protoplasm such as carbon, hydrogen, oxygen,
nitrogen, sulphur, iron and phosphorus are still the same in all the cells.
Question 9
What is the difference between an organ and an organelle?
SOLUTION 9
Organs of an organism are the parts of the body which have a definite shape
and structure and perform specific functions. Cell organelles are also parts
of the cell which have a definite shape and structure and perform specific
functions. Organelles have the same status in a cell as the organs have in the
entire body of an animal or a plant performing specific functions.
Question 10
Do you think the cells of an elephant would be larger than the cells of a rat?
Explain briefly.
SOLUTION 10
The cells of an elephant would be of the same size as the cells of a rat. The
size of cells does not vary within the organisms, however, the number of cells
varies from one organism to another. A larger animal like an elephant will
have more number of cells as compared to a smaller animal like a rat. However,
the size of the cell will be the same.
Question 11
Differentiate between the following pairs of terms:
(a) Protoplasm and cytoplasm
(b) Nucleolus and nucleus
(c) Centrosome and chromosome
(d) Cell wall and cell membrane
(e) Plant cell and animal cell
(f) Prokaryotes and eukaryotes
SOLUTION 11
(a) Protoplasm and cytoplasm
|
Protoplasm
|
Cytoplasm
|
|
(i) It is
the living matter, the total substance of a living cell, i.e. the
cytoplasm and the nucleus.
|
(ii) It is a
mixture of water and soluble organic and inorganic compounds, in which
various cell organelles are embedded.
|
(b) Nucleolus and nucleus
|
Nucleolus
|
Nucleus
|
|
(i) It is a
round-shaped nucleoli present inside the nucleus.
|
(ii) It is a
dense spherical structure present in the cell that contains a network
of thread-like structures called chromatin fibres.
|
(c) Centrosome and chromosome
|
Centrosome
|
Chromosome
|
|
(i) It is a
clear area of cytoplasm close to the nucleus, from which spindle
fibres develop during cell division.
(ii) Centrosome is found only in an animal cell.
|
(i)
Chromosomes carry hereditary information or genes which transmit
genetic characters from parents to offspring.
(ii)
Chromosomes are found in the nucleus of both, animal and plant cells.
|
(d) Cell wall and cell membrane
|
Cell wall
|
Cell membrane
|
|
(i) It is a non-living rigid layer.
|
(i) It is a living, thin, flexible membrane.
|
|
(ii) It is made of cellulose.
|
(ii) It is made of lipoproteins.
|
|
(iii) It is freely permeable.
|
(iii) It is semi-permeable.
|
(e) Plant cell and animal cell
|
Plant cell
|
Animal cell
|
|
(i) Cell wall is present.
|
(i) Cell wall is absent.
|
|
(ii) Centrosome is absent.
|
(ii) Centrosome is present.
|
|
(iii) Vacuoles are large and prominent.
|
(iii) Vacuoles are small and temporary.
|
|
(iv) Plastids are present.
|
(iv) Plastids are absent.
|
(f) Prokaryotes and eukaryotes
|
Prokaryotes
|
Eukaryotes
|
|
(i) Organisms with cells containing a primitive,
undefined nucleus are called prokaryotes.
|
(i) Organisms with cells containing a
well-defined nucleus with a nuclear membrane are called eukaryotes.
|
|
(ii) They contain small ribosomes.
|
(ii) They contain larger ribosomes.
|
|
(iii) They lack other cell organelles.
|
(iii) They contain other cell organelles.
|
|
(iv) Examples: Bacteria, blue-green algae
|
(iv) Examples: Euglena, Human beings
|
Question 12
Mention three features found only in plant cells and one found only in animal
cells.
SOLUTION 12
Features found only in plant cells:
(i) Presence of cell wall
(ii) Presence of large vacuoles. The liquid contained in vacuoles is called
cell sap
(iii) Presence of plastids
Features found only in animal cells:
(i) Presence of centrosome
Question 13
Why are the cells generally of a small size?
SOLUTION 13
Cells generally remain small in size because:
(i) To enable different regions of the cell to communicate with each other
rapidly for the cell to function effectively
(ii) To have a large surface area is to volume ratio for greater diffusion of
substances, in and out of the cell
Question 14
What is the cell theory? Who propounded it and when?
SOLUTION 14
Postulates of cell theory:
(i) Cell is the smallest unit of structure of all living things.
(ii) Cell is the unit of function of all living things.
(iii) All cells arise from pre-existing cells.
Cell theory was propounded by Theodor Schwann and Matthias Schleiden in the
year 1839 and was modified by Rudolf Virchow in 1858.
Question 15
Mention any three differences between a living cell and a brick in a wall.
SOLUTION 15
|
Living
cell
|
Brick
in a wall
|
|
1. Non-rigid living
structure
|
1. Rigid non-living
structure
|
|
2. Mainly composed
of cellulose
|
2. Mainly composed
of soil
|
|
3. Freely permeable
|
3. Impermeable
|
Question 16
Name the plastid and pigment likely to be found in
the cells of
(a) petals of sunflower
(b) ripe tomato
(c) skin of green mango
(d) cells of potato
SOLUTION 16
|
Cells
|
Plastid
|
Pigment
|
|
(a) petals of sunflower
|
Chromoplasts
|
Xanthophyll
|
|
(b) ripe tomato
|
Chromoplasts
|
Carotene
|
|
(c) skin of green mango
|
Chloroplasts
|
Chlorophyll
|
|
(d) cells of potato
|
Leucoplasts
|
No pigment
|
Question 17
State the major functions of the following:
(a) Plasma membrane
(b) Ribosome
(c) Lysosome
(d) Mitochondria
(e) Golgi apparatus
(f)
Cytoplasm
(g) Asters of centrosome
(h) Chromosomes
(i) Glycogen granule
(j) Vacuoles
SOLUTION 17
(a)
Plasma membrane:
(1) Separates contents of the cell from its surroundings
(2) Regulates the entry of certain solutes and ions
(3) Maintains the shape of animal cell
(b) Ribosome:
(1) Protein synthesis
(c) Lysosomes:
(1) Intracellular digestion
(2) Destroy foreign substances
(3) When the cell is old or injured, lysosomes rapidly destroy cell organelles
and hence, are called suicide bags.
(d) Mitochondria:
(1) Synthesis of respiratory enzymes
(2) Release of energy from pyruvic acid produced in cytoplasm in the form of
ATP
(e) Golgi apparatus:
(1) Synthesis and secretion of enzymes, hormones, etc.
(2) Formation of acrosome of sperm
(f) Cytoplasm:
(1) Different organelles contained in it perform different functions.
(2) All metabolic activities occur in it.
(g) Asters of centrosome:
(1) Initiates and regulates cell division
(2) Forms spindle fibres
(h) Chromosomes:
(1) Carry genetic characters from parents to offspring
(i) Glycogen granule:
(1) Serves
as food for the cell
(j) Vacuoles:
(1) Gives turgidity to the cells
(2) Storage of water and other substances, food, pigments and waste products
Question 18
List any six features found both in plant and animal cells.
SOLUTION 18
Common features found in both plant and animal cells:
(1) Presence of cell membrane
(2) Presence of liquid matrix called cytoplasm in the cell
(3) Presence of mitochondria which produces energy
(4) Presence of ribosomes that synthesize proteins
(5) Presence of Golgi body
(6) Presence of a prominent nucleus
Question 19
Given below are the sketches of two types of cells A and B.
(a) Which one of these is a plant cell? Give reason in support of your answer.
(b) List the cell structures which are common to both the types.
(c) Name the structures found only in plant cells and those found only in
animal cells.
SOLUTION 19
(a) Fig. B is a plant cell. It has a cell wall and a large vacuole which
pushes the nucleus towards the periphery.
(b) Cell membrane, ribosomes, nucleus, endoplasmic reticulum, lysosomes, Golgi
body and mitochondria are common to both the types.
(c) Plastids and cell wall are found only in plant cell. Centrosome is
found only in animal cell.
GEOGRAPHY
Earth’s Structure
Exercises
I. Short Answer Questions
Question 1. Name the sources of information about
forces operating inside the earth. Answer: Information about forces operating
inside the earth is taken by the study of seismic waves, materials thrown up
by volcanoes and the evidence from the theories of the origin of the earth.
Question 2. In which part of the earth is NIFE found
? What it is composed of ? Answer: NIFE is found in the inner core of die
earth. NIFE is composed of Nickel (Ni) and Iron (Fe), being heavy metals and
having high density, these are found deep inside the earth.
Question 3. What are the consequences of the pressure
and temperature in the interior of the earth ? Answer: Due to the extreme
temperature of 2200° C, every matter is in liquid and gaseous state in the
interior core, the temperature in the mantle ranges from 870° C to 2200° C, so
things are in semi – liquid to liquid state, the crust, a layer of 60 km,
breadth, is made of solid rocks with a density of 2 – 3, g/Cm3. The density
goes on increasing to 3-4g/Cm3 in the mantle and 10-13 g/Cm3 in the core.
Question 4. What is the lithosphere ? Answer: The
crust is called Lithosphere made of solid rocks with a thickness of 60 km.
below high mountains and 6-12 km below the oceans.
Question 5. Name the three layers of the earth’s
interior. Answer: The three layers of earth’s interior are:
1. Core 2. Mantle
3. Crust
Question 6. State two chief characteristics of the
earth’s crust. Answer: The crust is made of solid rocks and divided into
oceans and continents.
Question 7. Describe the mantle. State its two chief
characteristics. Answer: Mantle lies between 60 – 2900 km. depth. It is
divided into two parts namely upper mantle and lower mantle, which are in the
form of solid rocks and semi-molten rocks respectively.
Question 8. Where is asthenosphere found ? In which
form does it exist ? Answer: At the depth of 100 – 250 km. The mantle is
partially molten and known as asthenosphere, with a temperature of 1100°C.
Question 9. Write one difference between Moho
Discontinuity and Gutenberg Discontinuity. Answer: Moho Discontinuity is the
boundary between crust and mantle and Gutenberg Discontinuity is the boundary
between mantle and core.
Question 10. Why is the earth’s interior in most part
found in a solid state despite great heat and pressure ? Answer: The solid
state of the inner core is due to high density and pressure which have
compressed molten rock material and keep this layer firm and solid in some
parts due to high pressure inspite of the temperature of 5000°C.
Question 11. Name two types of earth movements.
Answer: Two types of movements are due to isostasy and tectonic plates.
Isostasy is the process of natural balance between different landforms and the
sliding movement of the tectonic plates of the earth’s crust.
Question 12. What is Geology ? Answer: Geology is the
science dealing with the origin and types of rocks found in the interior of
the earth.
II. Give reasons for each of the following
Question 1. The study of meteorites helps scientists
to know about the interior of the earth. Answer: At the time of the origin of
the earth every planet and meteorites were floating in the space and the
materials of the earth were same as that of the meteorites. So, the scientists
can calculate the composition of rocks ofthe earth by studying the materials
found in the meteorites.
Question 2. Temperature starts rising gradually
towards the interior of the earth. Answer: Due to enhancing density and
pressure the temperature goes on increasing gradually towards inside of the
interior of the earth.
Question 3. The asthenosphere is in a semi-molten
state. Answer: At the depth of 100 – 250 km the mantle is partially molten and
known as asthenosphere due to the temperature around 1100°C along with greater
pressure and density.
Question 4. The inner core is in a solid state.
Answer: The high pressure in the interior core keeps this layer firm and solid
in some parts, in spite of the temperature as high as 5000°C.
Question 5. The continents are placed above the
oceans. Answer: The density of continents is lesser than the layer supporting
the ocean beds, so the continents came floating upwards at the time of the
formation and solidification of the earth, as the lighter things come upwards
floating over the heavier things i. e. Sial is lighter than Sima
III. Long Answer Questions
Question 1. Look at the figure on the side and answer
the questions:
(a) Label the parts : (1), (2), (3), (4) and (5). (b)
Name the state (solid, liquid or gas) in which each part exists.
Answer: (a)
1. Atmosphere 2. Lithosphere 3. Mantle 4. Core 5.
Hydrosphere.
(b)
1. Atmosphere — Gas 2. Lithosphere — Solid 3. Mantle
— Semi-solid 4. Core — Molten state or liquid 5. Hydrosphere — Liquid (water)
(c) What part is suitable for human habitation? Why?
Ans. Outer part of the earth is suitable for human habitation due to
favourable conditions for survival, i.e. atmosphere for air, Lithosphere for
settlement due to ideal temperature and land, hydrosphere for hydrological
cycle for providing rainfall and fresh water bodies on the earth and oceans
for navigation and trade routes etc.
Question 2. Describe the layers of the interior of
the earth and their chemical composition. Answer: The interior of the earth is
divided into three major parts i.e. crust, mantle and core. The crust consists
of majority of, silica and aluminium and is called ‘SIAL’, mantle is called
SIMA due to the majority of silica and magnesium and is called SIMA, which
makes the bed of oceans and the core is called NIFE with excess of Nickel and
Iron (Ni + Fe).
Question 3. There are two transitional zones between
the two consecutive layers of the interior of the earth. Name them and state
their chief characteristics. Answer: The transitional zone between crust and
mantle is Moho Discontinuity which is the dividing zone between solid and
semi-solid state of rocks due to the increasing temperature as the depth
increases. The boundary between mantle and core is known as Gutenberg
Discontinuity, below this zone both density and temperature going on
increasing. The density is more than 13 and temperature is more than 2200°C.
Question 4. Explain the layers of the interior of the
earth with reference to the following :
a) Depth, (b) Temperature (c) Density.
Answer:
Crust :
(a) Depth 35-50 km below continents and 6-12 km.
below the oceans. (b) Temperature — Less than 870°C (c) Density — 2.7 g/Cm3 –
5.5 g/Cm3.
Mantle :
(a) Depth — 50 – 2900 km. (b) Temperature — 1500° C –
2200°C (c) Density — 3 g/Cm3 – 4.5 g/Cm3
Core :
(a) Depth — 2900 km – 3500 km (b) Temperature —
2200°C – 5000°C (c) Density — 10.0 g/Cm3 – 13.6 g/Cm3.
Question 5. Study the figure on the side and answer
the questions:
(a) What is known as Sial ? How deep is the area
marked by Sial ? (b) What role does Sima play ? (c) Why is the expression
‘Nife’ so called ? (d) Which layer is responsible for earth’s magnetic field ?
Why? (e) What happens to the continents if there is an earthquake?
Answer:
(a) Sial is the upper layer or crust of the earth.
The name Sial is based on the excess of Silica and Aluminium. The depth of
Sial is 60 km. (b) Sima is the second layer of earth which gives support to
the ocean beds. There is the excess of Silica and Magnesium. (c) ‘Nife’ means
Nickel (Ni) and Iron (Fe) due to the majority of Nickel and Iron in the core,
(d) Core of earth is responsible for earth’s magnetic field because it is
composed of iron and nickel which is responsible for earth’s magnetism.
Magnetic field is oriented towards North and South Poles. (e) Several drastic
changes may occur during the earthquake. It depends on the intensity of the
earthquake. If intensity is 8 or more buildings may break up, casualties and
major changes on the landforms as broad breaking gaps, origin or disappearing
of several small islands.
Practice Questions (Solved)
Question 1. Which are the two most abundant chemical
elements in the Earth’s crust ? Answer: Oxygen and Silicon.
Question 2. Why does the Sun not rise at the same
time everywhere in the world ? Answer: If the Earth were flat, the whole world
would have the sunrise and sunset at the same time. As the Earth is spherical
and rotates from West to East, places in the East see the Sun earlier than the
places in the West.
Question 3. “The whole of the approaching ship is not
visible at one time.” Why ? Answer: The Earth has a spherical snape. Along its
curved surface, the appearance of a ship is gradual. We see first the smoke,
then the mast and then the hull. If the Earth were flat, the entire ship would
be seen all at once.
Question 4. “Even when the Earth is spherical, it
appears to be flat.” Discuss. Answer: The actual shape of the Earth is
spherical. The curvature of the Earth is small as compared to its big size.
For a small area upto 100 sq. miles, this curvature is negligible. Therefore,
it appears flat.
Question 5. Why is the Earth slightly flattened at
the poles ? Answer: The Earth is not a perfect sphere. It is slightly
flattened at both the poles. It is due to the centripetal force produced by
the rotation of the Earth.
Question 6. Explain briefly the structure of the
earth. OR Discuss the structure of the earth giving details about each of its
layers and arguments in support of your contention. Answer: The structure of
the earth means the interior of the earth. The entire earth is composed of
three zones
1. Lithosphere 2. Mesosphere and 3. Barysphere.
(i) The Crust or Lithosphere It is the outermost
layer. Its thickness varies from 8 to 60 kms. It is solid and is formed
largely of igneous rocks. The crust consists of two layers.
1. a lower, continuous layer of basaltic (mafic)
rocks and 2. and upper layer of granitic (felsic) rock, which constitutes bulk
of continents. It is absent in ocean basins. These parts of the crust forming
the continents are much thicker than the crust under the oceans. Its main
universal constituents are Silica and Aluminium. It is collectively known as
‘SIAL’. It has an average density of 2.7. The lower layer has an average
density of 3.0. It main mineral constituents are
Silica (SI) and Magnesium (MA) and is therefore
called ‘SIMA’. Since the SIAL is lighter than the SIMA, the continents can be
said to be floating on the layer of denser SIMA.
(ii) Mantle or The Mesosphere Beneath the crust or
Lithosphere lies the Mantle or Mesosphere. Its thickness is 2840 km. Its
density is 3.1 to 5.0. It depth is 2900 km. It is again divided into two sub
layers.
1. the internal Silicate layer (SIMA). Its thickness
is 1140 km and density varies from 3.1 to 4.75 and 2. Mixed layer of metals
and silicates. Its thickness is 1700 kms. and density is 4.75 – 5.0.
(iii) Core or the Barysphere It is the central
nucleus. It is made up of dense rock materials – Nickel and Iron. It is also
called the layer ‘NIFE’. Its thickness is 3471 km (radius of core). It is
again divided into two sub-layer
1. The outer core is liquid or plastic in nature and
2. Inner core (Barysphere) which is solid and rigid because of tremendous
overlying pressure. The density of core is 5.1 to 13.
Question 7. Where is Mantle located in the Earth ?
Answer: The Mantle or Mesosphere is located between 2850 – 2900 km beneath the
earth crust.
Question 8. Describe any three experiments to prove
the Spherical Shape of the Earth. Answer:
1. If you observe a ship approaching sea coast, the
top of the mast is seen first and the hull, lower parts are seen gradually.
Due to the curvature of the Earth, the whole ship is not seen at one time.
2. Fix three poles of equal length at equal distance
on the ground. These do not give a horizontal level. The top of the middle
pole looks higher than the other two poles due to the curvature pf the Earth.
This experiment was done by Mr A.R. Wallace
on Bedford canal.
3.
If you look around at the Earth’s horizon (where Earth and sky appear to
meet), it will everywhere and always appear circular. It widens with
increasing altitude due to Spherical Earth.
Biology standard 9. Chapter 4(The Flower)
A. MULTIPLE CHOICE TYPE
1. Bougainvillea flower is an example of
Ans(d). large colourful bracts
2. A flower is said to be complete when:
Ans(d). It has all the four whorls.
3. The part of the flower that gives rise to the fruit is
Ans(c). Ovary
4. The part of flower that gives rise to seed is
Ans(c). Ovule
5. The essential whorls of a flower are the
Ans(d). Androecium n gynoecium
B. Very short Answer type
1. Match
(a) Polyadelphous—— bombax
(b) pollen grain—— pollen sac
(c) free petals—— polypetalous
(d) Non-essential—— calyx,corolla
(e) sweet fragrant fluid—— nectar
C. Short Answer Type
1. Explain
(a) Incomplete Flower
Ans. If one or more sets of floral structures are missing, the flower is
called incomplete.
(b) Staminate flower
Ans. A unisexual flower which contains only the stamens is called
Staminate flower .
(c) Pistillate Flower
Ans. The flower which contains only the carpels is called pistillate
flower.
(d) Bisexual Flower
Ans. A flower which contains both stamens and carpels is called bisexual
flower.
3. Where are following structures/parts located and their function
(a) placenta—- ovary
Function—- attach ovule to the wall of ovary
(b) Thalamus—- tip of flower stalk
Function—- attach flower to the stalk
(C) Anther—- stamen
Function— contains pollen grains
(d) Stigma—- Carpel
Function—- landing place for pollen grain
4. Why are the following described as stated:
(a). The androceium of pea flower is diadelphous because filaments are
united in two bundles
(b). Ray florets of sunflower as neuters because both male and female
reproductive organs are lacking.
(C). Salvia sepals are petaloids because perianth is non green.
D. Long Answer Type
Ans(a). 1. Monadelphous: Stamen are united in one group by their filament.
E.g. china rose
2. Diadelphous: The filaments are united in two bundles. E.g. pea
3. Polyadelphous: the filaments are united in several groups. E.g. bombax
Ans(b). (a) China rose—- Monadelphous
(b) Bombax—- Polyadelphous
(C) pea—- diadelphous
ote : these
questions will include an inside portion.
Class 9
History and Civics
Chapter 1 of
civics
Question 1:
What is meant by the term constitution
Answer:
The Constitution is a comprehensive
document containing the set of rules according to which the government of a
country runs. It regulates the position and powers of three organs of the
government- the legislative, the executive and judiciary.
Question 2:
On the basis of which plan was the
constituent assembly constituted?
Answer:
Cabinet mission plan.
Question 3
What is objective resolution?
Answer:
Objective resolution
was proposed by pt. Jawahar lal nehru on december 13, 1946 for
highlighting the national goals.
Question 4;
By whom was the objective resolution
proposed?
Answer:
It was proposed by the Pt. Jawahar Lal
Nehru.
Question 5:
Who was appointed as the chairman of
the drafting committee of the constitution?
Answer
Dr. Bhim Rao Ambedkar was appointed as
the chairman of the Drafting committee.
Question 6:
When was the constitution adopted and
passed? When did it come into force?
Answer
- Adopted and
passed: 26 November 1949.
- Come into
force: 26 January 1950.
Question 7:
State the significance of January 26.
Answer :
It was on this date, January 26, in
1929, that the lahore session of the indian national congress had for the
first time given the call for purna swaraj. Since then, the day was celebrated
as independence day upto 1947, but later on, it was designated as the republic
day.
Question 8:
What is the preamble?
Answer :
The Preamble is the introductory part
of the constitution, which sets out the main objectives of the constitution.
Question 9;
Explain the significance of the term
sovereign.
Answer:
It means that India is its own supreme
power and not a subject of any other state or country.
Question 10.
Mention any two features indicating
the significance of the preamble.
Answer:
1.The preamble represents the essence,
the philosophy, the ideals of the entire constitution of india.
2. It contains the five basic features
of the constitution ie. sovereign, socialist, secular, democratic and
republic.
Question 11.
Who represented the anglo indians in
the constituent assembly /
Answer
Mr. frank anthony and S.H prater
Question 12.
Why is our constitution known as the
fundamental law of the land?
Answer
Being superior to the ordinary law of
the state, the constitution of India is known as the fundamental law of land.
Structured questions
Question 1
With the reference to the making of
indian constitution explain the following
Part a.
When and how……...elected?
Answer 1.
The first sitting of the assembly was
held on december 9, 1946
The cabinet mission plan arrived in
India in 1946 and put forward a set of proposals to meet the demands of
freedom fighters. One of these proposals involved setting up of a constituent
assembly whose members were to be elected
indirectly by the provincial legislative
assemblies.
Part b.
How was the
membership……..partition of the country?
Answer
The muslim league boycotted the
constituent assembly consequently the members representing the territories
went to pakistan withdrew from the
cosntituent assembly of india. As a result, the membership of the constituent
assembly of india stood at 299n against the original number of 385.
Part. c how can you say that
constituent …………….of the indian society.
Answer:
The wide ranging membership of the
constituent assembly gave representation to all shades of public opinion.it
was composed of members from all the categories and communities belonging to
india.
Question 2
With the reference to objective
resolution
Part a.
Same as the 4th answer.
Part b
Main points
Answer1.
- India will be a
republic.
- The ideals of
social, political and economic democracy would be guaranteed to all the
people.
- The republic
would grant fundamental rights to citizens.
- The state would
safeguard the rights of backward and minorities.
Part c.
Three principles
Answer:
- Making indian
constitution workable, flexible and strong enough to hold the country
together both in peace and in war.
- Providing
special safeguard to the minorities.
- Incorporating
the right to constitutional remedies.
Class 9
Chapter
4 (a) Physics
1. Define the term thrust
state its S.I.unit.
Ans:- Thrust is the force acting normally on a
surface.S.I.unit of thrust is newton (N).
2. What is meant by pressure?
state its S.I.unit.
Ans:- Pressure is the thrust per unit area of
surface.S.I.unit of pressure is pascal or Nm-2.
3. Define one pascal (Pa),
the S.I.unit of pressure.
Ans:- One pascal is the pressure exerted on a
surface of area 1m2 by a force of 1N acting normally on it.
4.
Differentiate between thrust and pressure.
Ans:-
Thrust
1. Thrust
is the force acting normally on surface.
2. Thrust is a vector quantity.
3. S.I.unit of thrust is newton (N).
Pressure
1. Pressure is the thrust
per unit area of surface.
2. Pressure is a scalar quantity.
3. S.I.unit
of pressure is pascal .
5. How does the pressure exerted by a thrust depend
on the area of surface on which it acts.Explain with a suitable example.
Ans:- Pressure is inversly proportional to area.Example:- The ends of nails or
pins are made pointed so that large pressure is
exerted through the pointed
ends and they can be driven into with a less effort.
6. Why is the tip of
an allpin made sharp?
Ans:- The tip of an allpin made sharp so that even a
small thrust may cause a great pressure.
7. Explain the following
(a)
It is easier to cut with a sharp knife than with a blunt one.
Ans:- It is
easier to cut with a sharp knife so that a small thrust may cause a great
pressure at the edges and cutting can be
done with the less efforts.
(b) Sleepers are laid below the rails.
Ans:- Wide wooden sleepers are
placed below the railway tracks so that the pressure exerted by the iron rails
on the ground
become less.
8. What is the fluid?
Ans:- A substance
which can flow is called fluid.Example:- Liquids and gases.
9. What do you
mean by fluid pressure?
Ans:- Pressure exerted by fluid is called fluid
pressure.
10. State three factors on which pressure at a point in a liquid
depends.
Ans:-(i) Depth of the point below the free surface (h)
(ii)
Density of liquid(p)
(iii) Acceleration due to gravity (g)
11. Write an
expression for the pressure at a point inside a liquid .Explain the meaning of
the symbols used .
Ans:-P=hpg
Where h is depth
P(rho) is density of
liquid
g is acceleration due to gravity.
12. How does the pressure at a
certain depth in sea water differ from that at the same depth in river water?
Explain your answer.
Ans:-The pressure at a certain depth in sea water is
more than that at the same depth in river water. The reason is that the
density of sea
water is more than density of river water.
13. Explain
why a gas bubble released at the bottom of a lake grows in size as it rises to
the surface of lake.
Ans:-The reason is that when the bubble is at the
bottom of lake,total pressure exerted on it is the sum of atmospheric pressure
and
pressure due to water column.As the gas bubble rises due to decrease in
depth the pressure due to water column dereases,so the
total pressure
exerted on the bubble decreases.According to Boyle's law, volume of bubble
increases due to decreases
in pressure,i.e.the bubble grows in size.
14. A dam has broader walls at the bottom than at the top .Explain.
Ans:-The reason is that the pressure exerted by a liquid increases with its
depth.To with stand a greater pressure a dam has broader walls
at the
bottom.
15. Why do sea divers need special protective suit?
Ans:-Because
in deep sea, the total pressure exerted on the diver's body is much more than
his blood pressure.To with stand great
pressure,he or she needs to wear a
special protective suit.
16. State the laws of liquid pressure.
Ans:-Following are the five laws of liquid pressure.
(1) Inside the
liquid,Pressure increases with increase in depth.
(2) In a stationary
liquid,pressure is same at all points on a horizontal plane.
(3) A liquid
seeks its own level.
(4) Pressure at same depth is different in different
liquid.
(5) Pressure is same in all directions about a point inside the
liquid.
17. State Pascal's law of transmission of pressure.
Ans:-Pascal's law states that the pressure exerted anywhere in a confined
liquid is transmitted equally and undiminishhed
in all directions
throughout the liquid.
18. Name two applications of Pascal's law.
Ans:-(1) Hydraullic jack
(ii) Hydraullic brakes.
19. Explain the
principle of a hydraullic macchine.Name two devices which work on this
principle.
Ans:-The principle is that a small force applied on a smaller
piston is transmitted to produce a large force on the bigger piston.
Devices: (1) Hydraullic press.
(ii) Hydraullic jack.
20. Write one use
of hydraullic press.
Ans:-(1) For pessing cotton bales and goods like
quilts and books.
Please learn and write on your copy.
Class 9
Chapter
4 Physics
1. What do you mean by
atmospheric pressure?
Ans:- The thrust exerted per unit area on the earth
surface due to column of air is called atmospheric pressure on the
surface
of earth .
2. Name the physical quantity which is expressed in the unit
"atm" state its value in pascal ?
Ans:- Atmospheric pressure,1 atm =
1.013X10"5 Pa.
3. We donot feel uneasy even under the enormous pressure of
atmosphere above as well as around us give reason.
Ans:- Because pressure
of our blood balances it .The bood pressure is slightly more than the
atmospheric pressure .
4. State two uses of barometer.
Ans:- 1.To
measure atmospheric pressure .
2.For weather forecasting
5. Why does
the liquid rise in a syringe when its piston is pulled up?
Ans:- when its
piston is pulled up the pressure inside the barrel below the plunger is much
less than the
atmospheric pressure acting on the liquid surface.As a
result the atmospheric pressure forces the liquid to rise up
in the syringe
.
6. What is a barometer ? How is a simple barometer constructed ?
Ans:-
An instrument used to measure the atmospheric pressure is called a barometer .
A simple barometer consists of a glass tube of about 1m length closed at one
end.Fill the tube with pure mercury and
invert into a trough of mercury in
such a way that open end of tube is well immersed in mercury in the trough and
tube stand vertical. Now the thumb is removed .
it is seen that the
mercury level in tube falls till its height above
the mercury level in
trough becomes h=76cm.
7. Give two reasons for the use of mercury as a
barometric liquid.'
Ans:- 1.The mercury neither wets nor sticks to the
glass tube,therefore it gives accurate reading.
2.It can easily be obtained
in a pure state.
8. Give two reasons why water is not a suitable barometric
liquid ?
Ans:- 1.The vapour pressure of water is high,so its vapours in the
vacuum space will make the reading in accurate .
2.Water sticks with the
glass tube and wets it.So the reading become inaccurate.
9. Mention two
demerits of a simple barometer ?
Ans:- 1.There is no protection for the
glass tube.
2.It is inconvenient to move the barometer from one place to
another i.e.It is not portable.
10. State two advantages of an aneroid
barometer over a simple barometer?
Ans:- 1.This barometer has no liquid .
2.It is light and portable therefore it can easily be carried from one place
to another.
11. State two factors which affects the atmospheric pressure as
we go up?
Ans:- 1.Height of air column .
2.Density of air.
12. Why
does a fountain pen leak at a high altitude ?
Ans:- When the pen is taken
at an altitude,the atmospheric pressure at this altitude is low.So the excess
pressure due to
air inside the rubber tube forces the ink to leak out.
13. Why does nose start bleeding on high mountains?
Ans:- At high altitude
since the the atmospheric is low,breathing becomes difficult and nose bleeding
may occur due to
excess of blood pressure over the atmospheric pressure .
14. What do the following indicate in barometer regarding weather?
(a)
gradual fall in mercury level.
Ans:- Possibility of rain.
(b) Sudden
fall in mercury level.
Ans:- Coming of storm or cyclone.
(c) gradual
rise in the mercury level.
Ans:- dry weather.
lass 9
Chapter 1 - The Language of Chemistry Exercise
Ex. 1(C)
Question
1
Calculate the molecular mass of
the following:
CuSO4·5H2O
Given atomic mass of Cu =
63·5, H = 1, O = 16, C=12, N = 14, Mg = 24, S = 32
Solution
1
Molecular mass of CuSO4.5H2O
63.5 + 32 + (4 × 16) + (10
× 1) + (5 × 16)
= 63.5 + 32 + 64 + 10 + 80
= 249.5
Question
2
Fill in the blanks:
a.
Dalton used symbol _____ for
oxygen _____ for hydrogen.
b.
Symbol represents _____ atom(s) of
an element.
c.
Symbolic expression for a molecule
is called _____. .
d.
Sodium chloride has two radicals.
Sodium is a _____ radical while chloride is _____ radical.
e.
Valency of carbon in CH4 is
_____ , in C2H6 _____, in C2H4
___ and in C2H2 is ____.
f.
Valency of Iron in FeCl2 is
_____ and in FeCl3 it is ____ .
g.
Formula of iron (ill) carbonate is
_____ .
Solution
2
a.
Dalton used symbol [O] for oxygen,[H] for hydrogen.
b.
Symbol represents gram atom(s)
of an element.
c.
Symbolic expression for a molecule
is called molecular formula.
d.
Sodium chloride has two radicals.
Sodium is a basic radical, while chloride is an acid radical.
e.
Valency of carbon in CH4 is 4,
in C2H64, in C2H44
and in C2H2 is 4.
f.
Valency of iron in FeCl2 is 2
and in FeCl3 it is 3.
g.
Formula of iron (III) carbonate is
Fe2[CO3]3.
Question
3
Complete
the following table.

Solution
3

Question
4
Sodium chloride reacts with silver
nitrate to produce silver chloride and sodium nitrate
a.
Write the equation.
b.
Check whether it is balanced, if
not balance it.
c.
Find the weights of reactants and
products.
d.
State the law which this equation
satisfies.
Solution
4
a. NaCl
+ AgNO3 → NaNO3 + AgCl↓
b. It is a balanced equation.
c. Weights of reactants: NaCl - 58.44, AgNO3 - 169.87
Weights of products: NaNO3
- 84.99, AgCl - 143.32
NaCl + AgNO3 → NaNO3 + AgCl
(23+35.5) + (108+14+48) →
(23+14+48) + (108+35.5)
58.5 + 170 → 85
+ 143.5
228.5 g → 228.5 g
d. Law of conservation of
mass: Matter is neither created nor destroyed in the course of a chemical
reaction.
Question
5
What
information does the following chemical equation convey?
Zn
+ H2SO4 → ZnSO4+ H2
Solution
5
This equation conveys the following information:
i.
The
actual result of a chemical change.
ii.
Substances
take part in a reaction, and substances are formed as a result of the
reaction.
iii.
Here,
one molecule of zinc and one molecule of sulphuric
acid react to give one molecule of zinc sulphate
and one molecule of hydrogen.
iv.
Composition
of respective molecules, i.e. one molecule of sulphuric
acid contains two atoms of hydrogen, one atom of sulphur
and four atoms of oxygen.
v.
Relative
molecular masses of different substances, i.e. molecular mass of
Zn = 65
H2SO4 =
(2+32+64) = 98
ZnSO4 = (65+32+64) =
161
H2 = 2
vi.
22.4
litres of hydrogen are formed at STP.
Question
6
What information do the following
chemical equations convey? Mg + 2HCl → MgCl2+ H2
Solution
6
This equation conveys the
following information:
i.
Magnesium
reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.
ii.
24
g of magnesium reacts with 2(1 + 35.5) = 73 g of hydrochloric acid to produce
(24 + 71), i.e. 95 g of magnesium chloride.
iii.
Hydrogen
produced at STP is 22.4 litres.
Question
7
What are polyatomic ions? Give two
examples.
Solution
7
A polyatomic ion is a charged ion
composed of two or more covalently bounded atoms. Examples: Carbonate (CO32-)
and sulphate (SO42-)
Question
8
Name the fundamental law that is
involved in every equation.
Solution
8
Fundamental
laws which are involved in every equation:
i.
A
chemical equation consists of formulae of reactants connected by a plus sign
(+) and arrow (→) followed by the formulae of products connected by the
plus sign (+).
ii.
The
sign of an arrow (→) is to read 'to form'. It also shows the direction
in which the reaction is predominant.
The
fundamental law followed by every equation is 'Law of Conservation of Mass'.
Question
9
What is the valency
of :
fluorine
in CaF2
Solution
9
Valency of fluorine in CaF2 is
-1.
Question
10
What
is the valency of :
sulphur
in SF6
Solution
10
Valency
of sulphur in SF6 is -6.
Question
11
What
is the valency of :
phosphorus
in PH3
Solution
11
Valency of phosphorus in PH3
is +3.
Question
12
What is the valency
of :
carbon
in CH4
Solution
12
Valency of carbon in CH4 is
+4.
Question
13
What is the valency
of :
nitrogen in the following
compounds:
(i)
N2O3
(ii) N2O5 (iii) NO2
(iv) NO
(ii)
Mno2
(2)Cu20 ( 3)Mg3N2
Solution
13
Valency of nitrogen in the given
compounds: (2)(1) Mn=4 (2) cu=1 (3)mg=2
i.
N2O3 = N is +3
ii.
N2O5
= N is +5
iii.
NO2 =
N is +4
iv.
NO =
N is +2
Question
14
Why should an equation be
balanced? Explain with the help of a simple equation.
Solution
14
According to the law of
conservation of mass, 'matter can neither be created nor can it be destroyed'.
This is possible only if the total number of atoms on the reactants side is
equal to the total number of atoms on the products side. Thus, a chemical
reaction should always be balanced.
e.g. KNO3 →
KNO2 + O2
In this equation, the number of
atoms on both sides is not the same, and the equation is not balanced.
The balanced form of this equation
is
2KNO3 → 2KNO2
+ O2
Question
15
Write the balanced chemical
equations of the following reactions. sodium hydroxide + sulphuric
acid → sodium sulphate + water
Solution
15
2NaOH + H2SO4
→ Na2SO4 + 2H2O
Question
16
Write
the balanced chemical equations of the following reactions. potassium
bicarbonate + sulphuric acid → potassium sulphate + carbon dioxide + water
Solution
16
2KHCO3 + H2SO4
→ K2SO4 + 2CO2 + 2H2O
Question
17
Write
the balanced chemical equations of the following reactions. iron + sulphuric acid → ferrous sulphate
+ hydrogen.
Solution
17
Fe
+ H2SO4 → FeSO4 + H2
Question
18
Write
the balanced chemical equations of the following reactions.
chlorine + sulphur
dioxide + water → sulphuric
acid + hydrogen chloride
Solution
18
Cl2
+ SO2 + 2H2O → H2SO4 + 2HCl
Question
19
Write
the balanced chemical equations of the following reactions.
silver
nitrate → silver + nitrogen dioxide + oxygen
Solution
19
2AgNO3 → 2Ag +
2NO2 + O2
Question
20
Write
the balanced chemical equations of the following reactions.
copper
+ nitric acid → copper nitrate + nitric oxide + water
Solution
20
3Cu + 8HNO3 →
3Cu(NO3)2 + 2NO + 4H2O
Question
21
Write the balanced chemical
equations of the following reactions.
ammonia
+ oxygen → nitric oxide + water
Solution
21

Question
22
Write
the balanced chemical equations of the following reactions.
barium
chloride + sulphuric acid → barium sulphate + hydrochloric acid
Solution
22
BaCl2 + H2SO4
→ BaSO4 + 2HCl
Question
23
Write
the balanced chemical equations of the following reactions.
zinc sulphide
+ oxygen → zinc oxide + sulphur dioxide
Solution
23
2ZnS + 3O2 → 2ZnO
+ 2SO2
Question
24
Write
the balanced chemical equations of the following reactions.
aluminium
carbide + water → aluminium hydroxide +
methane
Solution
24
Al4C3
+ 12H2O → 4Al(OH)3 + 3CH4
Question
25
Write
the balanced chemical equations of the following reactions.
iron pyrites(FeS2) +
oxygen → ferric oxide + sulphur
dioxide
Solution
25
4FeS2 + 11O2
→ 2Fe2O3 + 8SO2
Question
26
Write
the balanced chemical equations of the following reactions.
potassium
permanganate + hydrochloric acid → potassium chloride + manganese
chloride + chlorine + water
Solution
26
2KMnO4 + HCl → 2KCl + 2MnCl2 + 5Cl2 +
8H2O
Question
27
Write
the balanced chemical equations of the following reactions.
aluminium
sulphate
+ sodium hydroxide → sodium sulphate + sodium
meta aluminate + water.
Solution
27
Al2(SO4)3
+ 8NaOH → 3Na2SO4 + 2NaAlO2 + 4H2O
Question
28
Write the balanced chemical
equations of the following reactions.
aluminium + sodium hydroxide + water →
sodium meta aluminate + hydrogen
Solution
28
2Al + 2NaOH + 2H2O →
2NaAlO2 + 3H2
Question
29
Write
the balanced chemical equations of the following reactions.
potassium dichromate + sulphuric acid → potassium sulphate
+ chromium sulphate + water + oxygen.
Solution
29
2K2Cr2O7
+ 8H2SO4 → 2K2SO4 + 2Cr2(SO4)3
+ 8H2O + 3O2
Question
30
Write
the balanced chemical equations of the following reactions.
potassium
dichromate + hydrochloric acid → Potassium chloride + chromium chloride
+ water + chlorine
Solution
30
K2Cr2O7
+ 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
Question
31
Write
the balanced chemical equations of the following reactions.
sulphur
+ nitric acid → sulphuric acid + nitrogen
dioxide + water.
Solution
31
S + HNO3 → H2SO4
+ NO2 + H2O
Question
32
Write the balanced chemical
equations of the following reactions.
sodium chloride + manganese
dioxide + sulphuric acid → sodium hydrogen sulphate
+ manganese sulphate + water +
chlorine.
Solution
32
2NaCl + MnO2 + 3H2SO4
→ 2NaHSO4 + MnSO4 + 2H2O + Cl2
Question
33
Define
atomic mass unit.
Solution
33
Atomic mass unit (amu) is equal to one-twelfth the mass of an atom of
carbon-12 (atomic mass of carbon taken as 12).
Question
34
Calculate the molecular mass of
the following:
(NH4)2CO3
Given
atomic mass of Cu = 63·5, H = 1, O= 16, C = 12, N = 14, Mg = 24, S =
32
Solution
34
Molecular
mass of (NH4)2CO3
= (2 × 14) + (8 × 1) +
12 + (3 × 16)
= 28 + 8 + 12 + 48
= 96
Question
35
Calculate
the molecular mass of the following:
(NH2)2CO
Given atomic mass of Cu =
63·5, H = 1, O= 16, C = 12, N = 14, Mg = 24, S = 32
Solution
35
Molecular mass of (NH2)2CO
= (14 × 2) + (4 × 1) +
12 + 16
= 28 + 4 + 12 + 16
= 60
Question
36
Calculate the molecular mass of
the following:
Mg3N2
Given atomic mass of Cu =
63·5, H = 1, O = 16, C = 12, N = 14, Mg = 24, S = 32
Solution
36
Molecular mass of Mg3N2
= (3 × 24) + (2 × 14)
= 72 + 28
= 100
Question
37
Choose the correct answer from the
options given below.
Modern atomic symbols are based on
the method proposed by
i. Bohr
ii. Dalton
iii. Berzelius
iv. Alchemist
Solution
37
iii. Berzelius
Question
38
Choose the correct answer from the
options given below.
The number of carbon atoms in a
hydrogen carbonate radical is
i.
One
ii.
Two
iii.
Three
iv.
Four
Solution
38
i. One
Question
39
Choose the correct answer from the
options given below.
The formula of iron (III) sulphate is
i. Fe3SO4
ii. Fe(SO4)3
iii. Fe2(SO4)3
iv. FeSO4
Solution
39
iii. Fe2(SO4)3
Question
40
Choose the correct answer from the
options given below.
In water, the hydrogen-to-oxygen
mass ratio is
i.
1:
8
ii.
1:
16
iii.
1:
32
iv.
1:
64
Solution
40
i. 1:8
Question
41
Choose the correct answer from the
options given below.
The formula of sodium carbonate is
Na2CO3 and that of calcium hydrogen carbonate is
i. CaHCO3
ii. Ca(HCO3)2
iii. Ca2HCO3
iv. Ca(HCO3)3
Solution
41
ii. Ca(HCO3)2
Chapter 1 - The Language of
Chemistry Exercise Ex. 1(A)
Question
1
What is a symbol? What information does it convey?
Solution
1
A symbol is the short form which stands for the atom of a
specific element or the abbreviations used for the names of elements.
i.
It represents a specific element.
ii.
It represents one atom of an element.
iii.
A symbol represents how many atoms are present in its one
gram (gm) atom.
iv.
It represents the number of times an atom is heavier than
one atomic mass unit (amu) taken as a standard.
Question
2
Why
is the symbol S for sulphur, but Na for sodium and
Si for silicon?
Solution
2
In most cases, the first letter of
the name of the element is taken as the symbol for that element and written
in capitals (e.g. for sulphur, we use the symbol
S). In cases where the first letter has already been adopted, we use a symbol
derived from the Latin name (e.g. for sodium/Natrium,
we use the symbol Na). In some cases, we use the initial letter in capital
together with a small letter from its name (e.g. for silicon, we use the
symbol Si).
Question
3
Write the full form of IUPAC. Name
the elements represented by the following symbols:
Au, Pb,
Sn, Hg
Solution
3
The full form of IUPAC is
International Union of Pure and Applied Chemistry.
Names of the elements:
Au
- Gold
Pb - Lead
Sn - Tin
Hg - Mercury
Question
4
If
the symbol for Cobalt, Co, were written as CO, what would be wrong with it?
Solution
4
Co stands for Cobalt. If we write
CO, then it would mean that it is a compound containing two non-metal ions,
i.e. carbon and oxygen, which forms carbon monoxide gas.
Question
5
2H2
Solution
5
2H2 stands for two molecules of
hydrogen.
Question
6
What is meant by atomicity? Name
the diatomic element.
Solution
6
The number of atoms of an element
that join together to form a molecule of that element is known as its
atomicity.
Diatomic molecules: H2,
O2, N2, Cl2
Question
7
Explain the terms 'valency' and 'variable valency'.
Solution
7
i.
Valency
of Na is +1 because it can lose one electron.
ii.
Valency
of O is -2 because it can accept two electrons.
Variable valency:
It is the combining capacity of an element in which the metal loses more
electrons from a shell next to a valence shell in addition to electrons of
the valence shell.
Question
8
How are the elements with variable
valency named? Explain with an example.
Solution
8
If an element exhibits two different
positive valencies, then
i. for
the lower valency, use the suffix -OUS at the end
of the name of the metal
ii. for
the higher valency, use the suffix -IC at the end
of the name of the metal.
Example:

Question
9
Give
the formula and valency of:
a.
aluminate
………………… .
b.
chromate
………….…….. .
c.
aluminium
………………. .
d.
cupric …………………
.
Solution
9

Question
10
What
is the significance of formula?
Solution
10
Significance
of the molecular formula:
-
It represents both molecule and molecular mass of the
compound.
-
It represents the respective number of different
atoms present in one molecule of the compound.
-
It represents the ratios of the respective masses of
the elements present in the compound.
Question
11
What do you understand by the
following terms?
Acid radical
Solution
11
Acid
radical: The electronegative or negatively charged radical is called an acid
radical.
Examples: Cl-, O2-
Question
12
What do you understand by the
following terms?
Basic
radical
Solution
12
Basic radical: The electropositive
or positively charged radical is called a basic radical.
Examples: K+, Na+
Question
13
Select the basic and acidic
radicals in the following compounds.
a.
MgSO4
b.
(NH4)2SO4
c.
Al2(SO4)3
d.
ZnCO3
e.
Mg(OH)2
Solution
13

Question
14
Write chemical formula of the sulphate of Aluminium, Ammonium
and Zinc.
Solution
14
Valencies of aluminium,
ammonium and zinc are 3, 1 and 2, respectively.
The valency
of sulphate is 2.
Hence, chemical formulae of the sulphates of aluminium,
ammonium and zinc are Al2(SO4)3, (NH4)2SO4
and ZnSO4.
Question
15
The valency
of an element A is 3
and that of element B is
2. Write the formula of the compound formed by the combination of A and
B.
Solution
15
Formula of the compound = A2B3
Question
16
Match the following:

Solution
16

Question
17
Write the basic radicals and
acidic radicals of the following
and then write the chemical
formulae of these compounds.
a.
Barium sulphate
b.
Bismuth
nitrate
c.
Calcium bromide
d.
Ferrous sulphide
e.
Chromium sulphate
f.
Calcium silicate
g.
Potassium ferrocyanide
h.
Stannic
oxide
i.
Magnesium phosphate
j.
Sodium zincate
k.
Stannic
phosphate
l.
Sodium thiosulphate
m.
Potassium manganate
n.
Nickel bisulphate
Solution
17
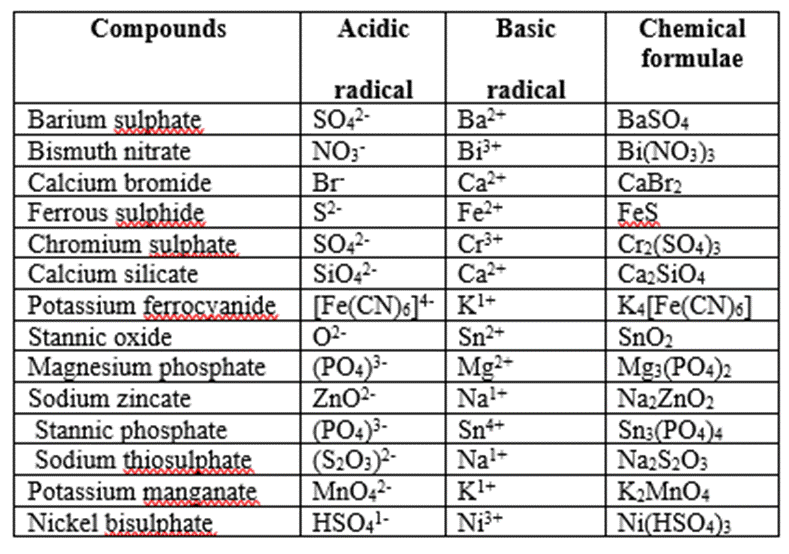
Question
18
Write
the chemical names of the following compounds:
a.
Ca3(PO4)2
b. K2CO3
c. K2MnO4
d.
Mn3(BO3)2
e.
Mg(HCO3)2
f.
Na4Fe(CN)6
g.
Ba(ClO3)2
h.
Ag2SO3
i.
(CH3COO)2Pb
j.
Na2SiO3
Solution
18
Chemical names of compounds:
a.
Ca3(PO4)2
- Calcium phosphate
b. K2CO3 -
Potassium carbonate
c. K2MnO4 -
Potassium manganate
d.
Mn3(BO3)2
- Manganese (II) borate
e.
Mg(HCO3)2 -
Magnesium hydrogen carbonate
f.
Na4Fe(CN)6 -
Sodium ferrocyanide
g.
Ba(ClO3)2 -
Barium chlorate
h.
Ag2SO3 -
Silver sulphite
i.
(CH3COO)2Pb -
Lead acetate
j.
Na2SiO3 -
Sodium silicate
Question
19
Give
the names of the following compounds.
a.
NaClO
b.
NaClO2
c.
NaClO3
d.
NaClO4
Solution
19
a.
NaClO - Sodium hypochlorite
b.
NaClO2 - Sodium
chlorite
c.
NaClO3 - Sodium
chlorate
d.
NaClO4 - Sodium
perchlorate
Question
20
Complete the following statements by selecting the correct
option :
The formula of a compound
represents
i. an
atom
ii. a
particle
iii. a
molecule
iv. a
combination
Solution
20
iii. The formula of a compound
represents a molecule.
Question
21
Complete the following statements
by selecting the correct option :
The correct formula of aluminium oxide is
i. AlO3
ii. AlO2
iii. Al2O3
Solution
21
iii. The correct formula of aluminium oxide is Al2O3.
Question
22
Complete the following statements
by selecting the correct option :
The valency
of nitrogen in nitrogen dioxide (NO2) is
i.
one
ii.
two
iii.
three
iv.
four
Solution
22
iv. The valency
of nitrogen in nitrogen dioxide (NO2) is four.
Question
23
Give
the names of the elements and number of atoms of those elements present in
the following compounds.
a.
Sodium sulphate
b.
Quick lime
c.
Baking soda (NaHCO3)
d.
Ammonia
e.
Ammonium dichromate
Solution 23
a. Sodium sulphate 2SO4
There
are two sodium atoms, one sulphur atom and four
oxygen atoms.
b.
Quick lime - CaO
There is one calcium atom and one oxygen atom.
c.
Baking soda - NaHCO3
There is one sodium, carbon and hydrogen atom and three oxygen atoms.
d.
Ammonia - NH3
There is one nitrogen atom and three hydrogen atoms.
e.
Ammonium dichromate - (NH4)Cr2O7
There two ammonium atoms, two chromium atoms and seven oxygen atoms.
Question 24
The
formula of the sulphate of an element M is M2(SO4)3
Write
the formula of its
a.
Chloride
b.
Oxide
c.
Phosphate
d.
Acetate
Solution
24
The
valency of metal M is 3. So, the formulae are as
follows:
a.
Chloride - MCl3
b.
Oxide - M2O3
c.
Phosphate - M(PO4)
d.
Acetate - M(CH3COO)3
Chapter 1 - The Language of
Chemistry Exercise Ex. 1(B)
Question
1
What
is a chemical equation? Why it is necessary to balance it?
Solution
1
A
chemical equation is the symbolic representation of a chemical reaction using
the symbols and formulae of the substances involved in the reaction.
A
chemical equation needs to be balanced because a chemical reaction is just a
rearrangement of atoms.
Atoms
themselves are neither created nor destroyed during the course of a chemical
reaction.
The
chemical equation needs to be balanced to follow the law of conservation of
mass.
Question
2
State
the information conveyed by the following equation:
Zn(s)
+ 2HCl(aq) → ZnCl2(aq) + H2 ↑
Solution
2
A
solid metal zinc reacts with hydrochloric acid in the aqueous state to
produce zinc chloride in the aqueous state and hydrogen gas.
Question
3
What
is the limitation of the reaction given in question 2?
Solution
3
-
The chemical equation given in question 2 does not
give the time taken for the completion of the reaction.
-
Also, it does not give information about whether heat
is absorbed or evolved during the reaction.
Question
4
Write
the chemical equations for the following word equations and balance them.
a.
Carbon +
Oxygen → Carbon dioxide
b.
Nitrogen + Oxygen → Nitrogen
monoxide
c.
Calcium + Nitrogen → Calcium
nitride
d.
Calcium oxide + Carbon dioxide →
Calcium carbonate
e.
Magnesium + Sulphuric
acid → Magnesium sulphate + Hydrogen
f.
Sodium reacts with water to form
sodium hydroxide and hydrogen
Solution
4
a.
C + O2→ CO2
b. N2 + O2→
2NO
c.
3Ca + N2→ Ca3N2
d.
CaO + CO2→ CaCO3
e.
Mg + H2SO4→
MgSO4 + H2↑
f.
Na + H2O → NaOH + H2↑
Question
5
Balance
the following equations:
a. Fe
+ H2O → Fe3O4 + H2
b. Ca
+ N2 → Ca3N2
c. Zn
+ KOH → K2ZnO2 + H2
d. Fe2O3
+ CO → Fe + CO2
e. PbO + NH3 → Pb
+ H2O + N2
f. Pb3O4
→ PbO + O2
g. PbS + O2 → PbO
+ SO2
h. S
+ H2SO4 → SO2 + H2O
i. S
+ HNO3 → H2SO4 + NO2 + H2O
j. MnO2
+ HCl → MnCl2 + H2O +
Cl2
k. C
+ H2SO4 → CO2 + H2O + SO2
l. KOH
+ Cl2 → KCl + KClO
+ H2O
m. NO2
+H2O → HNO2 + HNO3
n. Pb3O4
+ HCl → PbCl2 + H2O +
Cl2
o. H2O
+ Cl2 → HCl + O2
p. NaHCO3
→ Na2CO3 + H2O + CO2
q. HNO3
+ H2S → NO2 + H2O + S
r. P
+ HNO3 → NO2 + H2O + H3PO4
s. Zn
+ HNO3 → Zn(NO3)2
+ H2O + NO2
Solution
5
Balanced chemical equations:
Solution
5
Balanced chemical equations:
a.
3Fe + 4H2O → Fe3O4
+ 4H2
b.
3Ca + N2 → Ca3N2
c.
Zn + 2KOH → K2ZnO2
+ H2
d.
Fe2O3 + 3CO →
2Fe + 3CO2
e.
3PbO + 2NH3 → 3Pb
+ 3H2O + N2
f.
2Pb3O4 →
6PbO + O2
g.
2PbS + 3O2 → 2PbO
+ 2SO2
h.
S + 2H2SO4 →
3SO2 + 2H2O
i.
S + 6HNO3 → H2SO4
+ 6NO2 + 2H2O
j.
MnO2 + 4HCl →
MnCl2 + 2H2O + Cl2
k.
C + 2H2SO4 →
CO2 + H2O + SO2
l.
2KOH + Cl2 → KCl + KClO + H2O
m.
2NO2
+ H2O → HNO2 + HNO3
n.
Pb3O4 + 8HCl
→ 3PbCl2 + 4H2O + Cl2
o.
2H2O + 2Cl2 →
4HCl + O2
p.
2NaHCO3 → Na2CO3
+ H2O + CO2
q.
2HNO3 + H2S →
2NO2 + 2H2O + S
r.
P + 5HNO3 → 5NO2
+ H2O + H3PO4
s.
Zn + 4HNO3
→ Zn(NO3)2 + 2H2O + 2NO
HOME
CLASS - 9 - ENGLISH LANGUAGE
Book - Total English-9
1. An
CHAPTER
- 1 (Already solved in the book)
CHAPTER
- 2
ASSIGNMENT
(pg. 36)
Answers:
(A)
2. A____x
3. an
4. x_____x
5. a_____a
6.x_____x_____a
7. a_____x
8. an
9. a
10. a
(B)
1. The
2. The_____x
3. the_____the
4. The_____the
5. The_____the
6. x________the
7. x________a
8. The______the
9. The_______x_______the
10. The______x
Ques 5 (a)
1. Made
2. could resist
3. to trickle
4. had built
5. perfected
6. building
7. taking
8. sharing
(b)
1. with
2. across
3. at
4. down
5. through
6. about
7. up
8. from
(c)
1. I will find him wherever he goes.
2. Here is the lady whose daughter is a tennis
player.
3. The thief escaped in the dark.
4. She came to school though she had high fever
today.
(d)
1. You had better to come back tomorrow.
2. It seemed that he was warming up.
3. The musician’s performance on the stage
was admirable.
4. It was dangerous to cross the railway lines to
get to the station.
5. No sooner had the chief guest arrived than we
all stood up.
OR
No sooner did the chief guest arrive than we all
stood up.
6. Having featured his leg, Satish was forbidden
to go out by the doctor.
7. She invited us for dinner on Sunday.
OR
She invited us to have dinner with her on Sunday.
8. People say that he has been in the Indian
Army.
CHAPTER- 3
ASSIGNMENT
(pg. 53)
1.
Are
2.
Was
3.
Was
4.
Has
5.
Wait
6.
Is
7.
Deserve
8.
Is
9.
Is
10.
make
11. is
12.was
Word
Economy (pg. 56)
1. inimitable
2. inarticulate
3. inaudible
4. incombustible
5. impassable
6. incommunicative
7. Impotent
8. indelible
9. incorrigible
10. inaccessible
Ques
5(pg. 64)
(a)
1. invented
2. connecting
3. produced
4. to create
5. found
6. burnt
7. experimented
8. helped
(b)
1. from
2. in
3. out
4. up
5. Through
6. up
7. at.
8. across
(c)
1. The police will soon find out the snatcher of
the lady’s purse.
2. He does not know that there is something in
store for him.
3. It was careless of me to forget my passport.
4. She has to tell her mother about her going
there.
(d)
1. It is compulsory for us to study a third
language up to class VIII.
2. My uncle is so old that he cannot learn new
things.
3. Upon arriving I had a cup of tea.
4. He aims at becoming rich therefore he works
hard.
5. His music must be listened to by us.
6. The harsh weather prevented the ship from
sailing / to sail.
7. My shirt was washed before it was returned.
8. He asked Uma if she was going for the picnic
the next day.
CHAPTER 4
ASSIGNMENT
(pg 69)
1. have been
2. has____entered
3. have been fasting
4. has not visited
5. have been doing
6. have been waiting
7. has_____announced
8. has already written
9. has been talking
10. have finished
Ques 5.
(a)
1. to send
2. attacked
3. was surrounded
4. could manage
5. built
6. thought
7. hidden
8. destroyed
(b)
1. up
2. up
3. after
4. back
5. upon
6. down
7. to
8. against
(c)
1. The train will stop if you pull the chain.
2. This was what she said.
3. As I have completed my studies, I am looking
for a job
OR
Having completed my studies, I am looking for a
job.
4. We can travel either by bus or train.
(d)
1. After dressing up, Rima had her breakfast.
2. The little girl hid herself lest she should be
seen
3. The officer examined the documents with care.
4. Neelam exclaimed
that the sun looked very beautiful that day.
5. The last time I missed a match was 2 years
ago.
6. Beena is the most
melodious singer in the world.
7. Not until you pay the money you will not get
the camera back.
8. John isn’t as clever as handsome he is.
CHAPTER 5 (pg 94)
Ques. 5
(a)
1. race / run
2. were placed
3. allowed
4. was called
5. were added
6. went
7. throw
8. were revived
(b)
1. with
2. upon
3. at
4. of
5. over
6. in
7. out
8. through
(c)
1. Nobody has an idea what happened in the
meeting.
2. Take a check as the cash may not be
sufficient.
3. The book is so simple that even a child can
read it.
4. Your phone call came after I had left the
house.
(d)
1. Sunita was cycling
so fast that she could not stop at once.
2. It was stupid of me to forget his name.
3. I am so tired that I cannot walk any further.
4. Tara asked her father if she could have a new
dress.
5. People knew him to be a kind man.
6. She ought to have left earlier.
7. Unless we like the book, we won’t buy
it.
8. Besides treating him well he helped him too.
|

